Biomedical Engineering Reference
In-Depth Information
The drugs and combinations above are suitable for
initial immobilization and, with the addition of appropriate
local anesthetic techniques and/or
repeat dosing,
the
performance of minor surgical procedures.
Monitoring During the Immobilization Period
Once the animal has been immobilized the assessment of
the depth of anesthesia and the monitoring of physiological
parameters is important in order to ensure that the anes-
thetic level is deep enough to facilitate the required
procedure, but not so deep as to cause unnecessary physi-
ological impairment. It is not the case that sedation or light
anesthesia is inherently safer than surgical anesthesia and
therefore monitoring is less important. If the period of
immobilization required is very brief, such as for tubercu-
losis testing, blood withdrawal, or physical examination,
the connection of multiple electronic monitors at this time
may be ergonomically difficult. However, reflexes can be
tested, the color of mucous membranes and respiratory rate
observed, and pulse rate palpated (e.g. at the femoral artery
on the medial aspect of the upper hind limb). It is also
relatively simple to place a pulse oximeter on a digit, to
ensure hypoxia does not occur, and to use a rectal ther-
mometer. It is particularly useful to monitor physiological
trends at this stage to ascertain whether the depth of
anesthesia is increasing or decreasing as the drugs used for
immobilization continue to take effect. If the period of
immobilization is longer (
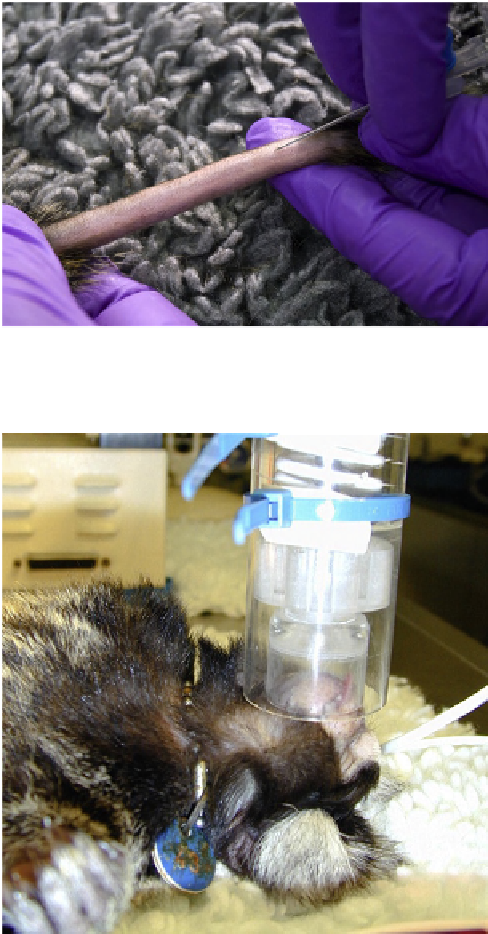
FIGURE 17.1
Indwelling “over the needle” cannula poised for
placement in the tail vein of a marmoset.
10 min) and/or minor surgical
procedures are to be performed, then the combination of
manual and more extensive electronic monitoring can be
very helpful. See “Intraoperative monitoring” below for
more details.
>
Deep (Surgical) or Prolonged General
Anesthesia
Awide range of anesthetic agents can be used successfully,
and in most species, following initial sedation/immobili-
zation, intravenous cannulas can be placed allowing
induction and maintenance of anesthesia with injectable
agents. The tail vein in marmosets and the cephalic and
saphenous vein in larger primates are easily visible and
placement of an “over-the-needle” cannula is relatively
straightforward (see “Intravenous cannula placement”
below) (
Figure 17.1
). In marmosets and tamarins, alphax-
alone intramuscularly, followed by additional doses intra-
venously or administration of inhalational agents by
face-mask (
Figure 17.2
), provides safe and stable anes-
thesia. The use of induction chambers or mask induction for
small nonhuman primates, particularly if nonpungent
inhalational agents (such as sevoflurane) are available, is
also possible. In larger primates, after initial sedation and
restraint with ketamine, anesthesia can be induced by
FIGURE 17.2
Administration of a volatile anesthetic agent to
a marmoset via a coaxial face-mask. The volatile agent, carried in
oxygen with or without nitrous oxide, is supplied at the desired concen-
tration via the inner (corrugated) tube and waste gas is scavenged via the
outer tube. (Courtesy of P. M. Taylor.)
intravenous administration of propofol or with volatile
anesthetics administered by face-mask. After endotracheal
intubation (see below), anesthesia can then be maintained
using an infusion of injectable anesthetics or inhalational
agents. Further information concerning regimens for pro-
longed anesthesia is given below.
The goals of anesthesia include maintaining a steady
state and avoiding excessive physiological impairment.
These goals are best achieved when the drug(s) are
administered continuously, either intravenously or by
inhalation. When drugs are administered intermittently,