Biomedical Engineering Reference
In-Depth Information
attempted from a donor animal of the same species without
a cross-match. In most cases, the risk of life-threatening
immunological reaction is minimal if it is the first time the
animal has received a transfusion even if the donor and
recipient are mismatched.
Once a need for transfusion has been established, the
goal should be to provide volume resuscitation via crys-
talloids and/or colloids and whole blood or pRBCs until the
hematocrit is 25 or greater (
Winberg, 2009
). Whole blood is
collected using aseptic technique into a collection syringe
that contains an anticoagulant. Most whole blood donor
collection reservoir kits used in veterinary medicine extract
too much volume and cannot be used directly for the most
commonly used species of nonhuman primates. If these
commercial systems are to be used, the anticoagulant must
be removed from the system and then added back at the
correct volume to match the collected blood volume.
Anticoagulants that may be used include acid-citrate-
dextrose (ACD) at a ratio of 1:9 with whole blood, heparin
at 10 units/ml whole blood, or 3.8% citrate at a ratio of 1:9
if transfusion will immediately follow collection (
Brainard,
2009
; California National Primate Research Center
(CaNPRC), 2009). For healthy donors who have not had
blood collected within the previous 30 days, 10 ml whole
blood/kg body weight can be collected safely.
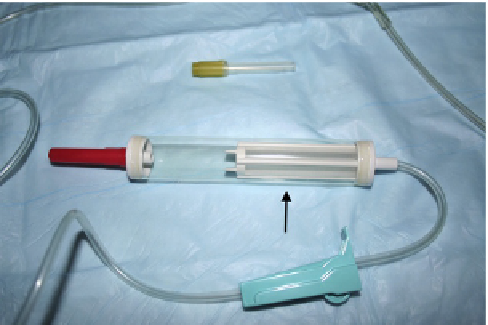
Blood is administered to the recipient aseptically via
a standard blood filter line so as to prevent administration of
clots (
Figure 15.3
). Filters are also available that can be
attached to intravenous administration lines. Blood should
be administered at a rate of 1 ml/kg for the first 15 minutes
and at a maximum rate of 22 ml/kg/h thereafter (
Brainard,
2009
). Temperature, pulse, and respiratory rate are assessed
prior to administration and at regular and frequent intervals
during administration to detect adverse reactions. Adverse
reactions are immune-mediated and may include urticaria,
and pruritus if mild, or collapse, tremors, tachycardia, and
death if severe (
Brainard, 2009
). In addition, animals
should be monitored for several hours post transfusion for
clinical signs of acute respiratory distress syndrome
(ARDS) and supported with oxygen if respiratory signs
develop. Other supportive therapy (antihistamine, cortico-
steroids, intravenous fluids) should be instituted as neces-
sary if adverse reactions occur.
Cardiopulmonary Cerebral Resuscitation
Cardiopulmonary arrest (CPA) is characterized by the
sudden cessation of spontaneous and effective circulation
and ventilation. The diagnosis of CPA is based on the
absence of effective ventilation, severe cyanosis, absence
of a palpable pulse or apex heartbeat, absence of heart
sounds, and ECG evidence of asystole or other non-
perfusing rhythm such as pulseless electrical activity
(PEA; formerly referred to as electromechanical dissoci-
ation), pulseless ventricular tachycardia, or ventricular
fibrillation. If the primary disease state causing cardio-
pulmonary arrest is reversible, prompt assessment and
intervention focused on maintaining circulation may save
the animal's life. In recent years, it has been acknowl-
edged that maintenance of cerebral circulation is as
important as cardiac perfusion (
Ford and Mazzaferro,
2005; Plunkett and McMichael, 2008; Wells, 2008
).
Cardiopulmonary cerebral resuscitation (CPCR) provides
artificial ventilation and circulation until advanced life
support can be provided or return of spontaneous circu-
lation (ROSC) occurs. Animals experiencing cardiopul-
monary arrest have historically had a poor prognosis, even
with appropriate intervention, and this may be the result of
underlying disease processes that exist. In species of
nonhuman primates with cardiomyopathy including Aotus
and macaque species, the underlying disease process
results in poor response to resuscitation efforts and a poor
prognosis. In veterinary medicine, even with aggressive
treatment and management, the overall success of CPCR
is less than 5% in critically ill or traumatized patients and
20% to 30% in anesthetized patients (
Ford and Mazza-
ferro, 2005
). Diagnosis of the primary disease state will
help determine if CPCR is warranted. Considerations for
resuscitation of nonhuman primates should be consistent
with the approved experimental endpoints if animals are
assigned to research protocols.
In 2010 the American Heart Association (AHA) pub-
lished new guidelines for CPCR in humans (
Neumar et al.,
2010
). Highlights of the new guidelines include a greater
emphasis on chest compressions, avoidance of excessive
ventilation rates, and immediate resumption of compres-
sions after a single defibrillation. Many of the recommen-
dations are based on research in small animals (canine and
feline) and are pertinent to veterinary patients. Much of the
FIGURE 15.3
Whole blood administration set for transfusion. The
reservoir (arrow) contains a filter to remove blot clots as whole blood is
administered. Whole blood should never be administered using standard
intravenous fluid drip sets without filters.