Biomedical Engineering Reference
In-Depth Information
a
b
0,1
1
Solvent
CdSe
1E-3
1E-5
2 ZnS monolayers
0,1
2
CdSe
Solventå
1E-3
1E-5
2 ZnS monolayers
0,1
3
c
CdSe
Solvent
1E-3
1
1E-5
0 ML
2 ZnS monolayers
0,1
0,01
Solvent
4
CdSe
1E-3
1E-5
0,1
1 ML
2 ZnS monolayers
0,1
1E-3
5
1
1E-3
CdSe
2
1E-5
0,01
0
1
2
3
4
2 ML
4
Radial position R, nm
1E-4
3
5
3 ML
1E-3
1E-5
2
3
4
5
QD Core Diameter d
CdSe
[nm]
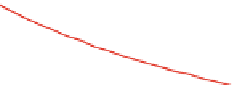
Fig. 4.21
General scheme of the QD PL quenching model and comparison of experimental
results and theoretical calculations for “QD-H
2
P” nanoassemblies: (
a
) QD-H
2
P interaction model.
(
b
) Logarithmic presentation of
2
(
r
) for a 1 s electron in a core-shell spherical potential for
five different sizes of QD with
n
ZnS
Ψ
0or2.(
c
) Comparison of the experimentally determined
quenching rate constants
k
q
(symbols with
error bars
,
left axis
) and calculated probability density
functions
=
2
(
r
D
)
between the ZnS shell and the environment as a function of core diameter d and ZnS shell thickness
D
. The constant
C
in equation
k
q
ψ
=
R
+
D
)(
lines
,
right axis
) of a 1 s electron at the outer interface (
r
=
R
+
2
2
(
R
(
r
)=
C
ψ
(
r
)
has been adjusted with respect to
ψ
+
D
)tofit
the experimental value at
d
CdSe
=
4.1 nm,
n
ZnS
=
2. All theoretical lines correspond to calculations
2
(
R
) with the same proportionality constant
C
. QD parameters are the same as in Fig.
4.20
.
Adapted from [
63
]
of
ψ
r
=
r
ji
=
r
=
r
ji
.
1
m
∗
i
d
dr
ψ
i
(
1
m
∗
j
d
dr
ψ
j
(
ψ
i
(
r
ji
)=
ψ
j
(
r
ji
)
and
r
)
r
)
(4.14)
2
(
r
)ofan
s
-type electron wave
function for four CdSe/ZnS QDs with two ZnS monolayers and one CdSe QD
without a ZnS monolayer. It can be seen that
Figure
4.21
b shows the calculated radial part of
ψ
2
(
r
) becomes smaller at the outer
interface (marked by a circle) upon increasing QD diameter. The corresponding
value is largest for the uncapped CdSe QD (
R
ψ
1.73 nm).
It is seen from Fig.
4.21
c that the comparison of rate constants
k
q
(symbols) and
the calculated (and scaled) probability densities
=
2
(
r
) (lines) shows a good corre-
lation. Thus, one point-like charge density perturbation caused by an organic linker
group or chromophore at the QD interface forces the electron of the delocalized
exciton of the QD to become localized. Qualitatively the same physicochemical
picture is characteristic also for “QD-PDI” nanoassemblies [
64
,
74
,
94
]. In fact, the
dependence of QD PL quenching induced by even only one single molecule on the
ψ