Biomedical Engineering Reference
In-Depth Information
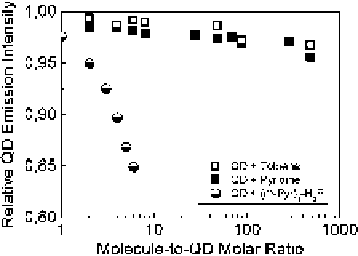
Fig. 4.16
2)
induced by (m-Pyr)
1
-H
2
P(
half circles
) and pyridine (
solid squares
).
Open squares
show the PL
quenching by addition of toluene only. Note that the PL change due to concentration change upon
addition of the aliquot is already taken into account. Adapted from [
123
]
Comparison of PL quenching of colloidal CdSe/ZnS QD (
d
CdSe
=
2.14 nm,
n
ZnS
=
PL quenching of the QD really needs the attachment of the H
2
P or PDI chromophore
or merely the pyridine substituent, a comparison has been performed of PL
quenching induced by titration with pyridine and with mono-pyridyl-substituted
porphyrin molecules (m-Pyr)
1
-H
2
P containing only one
meso
-pyridyl ring which
is necessary for the QD attachment (Fig.
4.16
). It turns out that an only small
(2-3%) decrease of the PL quantum efficiency of the QD is due to dilution by
toluene (open squares). Practically the same tendency is observed for titration
by pyridine (solid squares), which probably reflects a negligible quenching by
this molecule (pyridine is a hole acceptor like amines or thiols [
39
,
44
,
106
]).
In comparison, for molar ratios
x
1- 8, the PL quenching of QDs by mono-
pyridyl substituted porphyrin (m-Pyr)
1
-H
2
P is essentially stronger (half circles) in
comparison with the above two cases.
Several reasons for this behavior have to be considered. (1) Van-der-Waals
interactions between TOPO molecules and the (in comparison with pyridine) large
porphyrin molecule may favor a strong incorporation of a porphyrin molecule
into the QD interface (with respect to the QD surface perpendicular geometry,
see Fig.
4.7
). (2) Weak solubility of (m-Pyr)
1
-H
2
P in toluene may also favor a
fixation on the QD surface. (3) Electronic properties of the
=
-conjugated porphyrin
macrocycle having
meso
-pyridyl rings may play a specific role in the localization
of the electron-hole pair at the QD surface [
90
]. Concluding, these experiments
reveal that there is almost no PL quenching of CdSe/ZnS by pyridine even at 100
times increased relative molar ratios
x
. This observation prompts us to believe that
the porphyrin chromophore itself is of central importance although the specific
electronic structure is of much less influence [
101
]. Investigations on similarly
attached perylene diimide chromophores support this statement [
74
,
94
]. However,
we have in case of H
2
P dyes varied the electronic nature by fluorination of the
molecules without observing a pronounced influence on the PL quenching [
90
].
Interestingly, in the case of CdSe/ZnS QDs (
d
π
=
2.6 nm and
n
ZnS
=
2 monolayers),