Biomedical Engineering Reference
In-Depth Information
m
2
of
paclitaxel, measured over a 24-h period. The biphasic elimination can be clearly seen. Data were
taken from [
30
]
Fig. 7
A typical log-log plot of concentration versus time for a 3-h infusion of 225 mg
/
stabilizing microtubules [
54
]. Paclitaxel is well suited for pharmacokinetic studies
because it is administered at high doses, it has a long residence time in the body, and
it has been the subject of many dose escalation studies. It has shown a consistent
disproportional increase in the maximum plasma concentration (
C
max
)andthe
area under the curve (
AUC
)[
30
]. Following the end of the infusion, a biphasic
elimination has been noted [
27
].
In a meta-study of data from the literature, not only biphasic elimination, but
also a distinct power law relationship for each phase has been found. The first phase
begins immediately after the end of the infusion and consists of a rapid decline
lasting approximately 1 h. The subsequent prolonged terminal phase can last up to
72 h. Significantly, although the data span a wide range of age, sex, type of cancer,
total dose, and infusion time, the power exponents for the two elimination phases
seem to be approximately equal to
6, respectively. In addition, the time
period corresponding to uptake during the infusion also seems to obey a power law,
but with a much larger range of exponents. Figure
7
shows a representative log-log
plot for concentration-time data of paclitaxel.
Various reasons have been proposed in the literature for the source of the
nonlinearity of paclitaxel pharmacokinetics. The predominant explanation being
offered is the high binding of paclitaxel molecules to the Cremophor EL vehicle and
plasma proteins [
30
,
59
]. However, a related drug docetaxel, which does not have
Cremophor EL, has also shown nonlinear behavior [
68
]. In the study, 100 mg
−
3
.
3and
−
1
.
m
2
of
/
docetaxel was administered during a 6-h infusion. We found an exponent of
−
1
.
34
(
R
2
9830) for the period of 8-30 h. In addition, pharmacokinetic studies of ABI-
007, a Cremophor-free formulation of paclitaxel, demonstrated similar nonlinear
behavior. The data from one experiment [
49
] with ABI-007 produced an exponent
of
=
0
.
60 (
R
2
9469), while in another study [
14
] the area under the curve scaled
with dose with an exponent of
−
1
.
=
0
.
61 (
R
2
−
1
.
=
0
.
9477).

















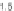


































Search WWH ::

Custom Search