Biomedical Engineering Reference
In-Depth Information
4.0e5
C
A
and
A-IS
E
2.0e5
F
and
F-IS
B
D
G
0.0
2.0
4.0
6.0
8.0
Time, min
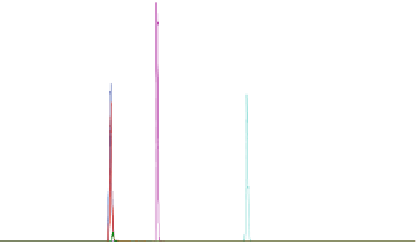
Fig. 1
Chromatogram of six compounds (Compounds A-F) with two labeled internal standards
(IS) and one nonlabeled IS. A-IS is the labeled IS for Compounds A and D and F-IS is the labeled
IS for Compound F. Compound G is the IS for Compounds C and E
2.5
Chromatography
Two mobile phases were used. Mobile phase A was 0.1 % formic acid and 5 % ace-
tonitrile in water and mobile phase B was 0.1 % formic acid and 95 % acetonitrile in
water. Chromatographic separation was achieved for all compounds using an 8.5 min
gradient method. Initially, 7.5 % mobile phase B was used for system equilibration.
After each injection, mobile phase B was run at 7.5 % for 1 min then it was linearly
increased to 90 % over the course of 3 min, it was maintained at 90 % for 2.5 min and
returned to 7.5 % in 0.1 min. The postgradient column equilibration was run for
1.9 min prior to the start of pretreatment for the next injection. Figure
1
shows a typi-
cal chromatogram of six compounds (Compounds A through F) and three internal
standards eluted with the described chromatography conditions. Compound G was
used as the internal standard for Compounds B, C, and E.
2.6
Mass Spectrometry
LC-MS/MS detection was performed using an AB Sciex API 4000
TM
triple quadru-
pole mass spectrometer equipped with a TURBO V
TM
ionspray ionization source
operated in positive ion mode. The software used for instrument control was Analyst
version 1.5. The spray voltage was set at 5,500 V and the source temperature was
550 °C. Other ion source parameter settings were 10 for curtain gas, 65 for GS1, 65
for GS2. The collision gas setting was 4 and the entrance potential was 10 V. Other
compound specific parameters such as the declustering potential (DP), the collision
energy (CE), and the cell exit potential (CXP) varied depending on the analyte. The
DP ranged from 50 to 110 V, the CE ranged from 23 to 80 eV, and the CXP ranged
from 5 to 15 V. Acquisition was performed in scheduled MRM mode with a 60 s
window around the retention time of each analyte and a target scan time of 0.2 s.