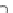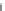Biomedical Engineering Reference
In-Depth Information
3
2
1
0
0
40
80
120
160
200
Sample Sequence No.
System Suitability Sample
CS and QC
Coded Incurred Sample
Normal Incurred Sample
Fig. 19
Randomly scattered low internal standard (IS) responses observed for incurred samples
only, whose IS responses were within normal range during repeat analyses. Analyte: olanzapine;
IS: olanzapine-d
3
; sample pretreatment at clinic: 25 % (w/v) L-ascorbic acid added to plasma in a
ratio of 1.25:100 (v/v); extraction: MCX (mixed-mode strong cation exchange)-based solid-phase
extraction. An incurred sample was coded for reassay when its IS response was outside ±50 % of
the mean IS response of the accepted calibration standards and quality controls. Reproduced from
ref. [
36
] with permission from Elsevier
samples due to interlot matrix differences, the same or similar IS responses would be
repeated during reassays. Without proper investigation or evaluation, either there
would be no reportable values for a whole subject (due to abnormal IS responses) or
there would be uncertainty on the accuracy of the results obtained if they are to be
reported. Both should be avoided during the analysis of incurred samples.
4
Conclusions
Internal standards play critical roles in ensuring the accuracy of final reported
concentrations in quantitative LC-MS bioanalysis through the correction of varia-
tions during sample preparation, LC-separation, and MS detection. The physical-
chemical properties of an internal standard, particularly hydrophobicity and
ionization properties should be as close as possible to those of the corresponding
analyte to better track the variations the analyte experiences. For this reason, stable
isotope labeled internal standards should be used whenever possible. However,
efforts should still be made to obtain clean extracts, adequate chromatographic
separation, and optimized ionization mode and conditions.