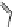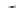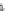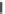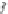Biomedical Engineering Reference
In-Depth Information
tertiary tropane alkaloids (TTA)
N
N
N
H
3
C
H
3
C
H
3
C
N
H
3
C
Cl
OH
OH
OH
O
HO
HO
O
O
O
O
Cl
O
O
O
O
anisodamine (Ada)
anisodine (Adi)
atropine (Atr)
bemesetron (Beme)
N
H
3
C
N
CH
3
O
O
H
3
C
N
N
O
OCH
3
H
3
C
H
3
C
N
OH
N
O
O
O
O
N
O
benzoylecgonine (Becg)
benztropine (Benz)
cocaine (Coc)
granisetron (Gran)
N
N
N
H
3
C
H
3
C
H
3
C
N
H
3
C
OH
OH
HO
OH
O
O
O
O
O
O
O
O
homatropine (Hatr)
R
-hyoscyamine (
R
-Hyo)
S
-hyoscyamine (
S
-Hyo)
littorine (Lit)
N
N
H
3
C
N
H
3
C
H
3
C
H
OH
O
H
3
C
O
O
O
O
S
O
O
O
CH
3
O
O
satropane (Sat)
S
-scopolamine (Scp)
tropisetron (Trop)
Quaternary tropane alkaloids (QTA)
CH
3
CH
3
CH
3
+
CH
3
+
+
+
N
H
3
C
N
H
3
C
N
H
3
C
N
H
3
C
OH
OH
OH
OH
O
O
O
O
O
O
O
O
O
O
O
N-butyl-scopolamine (BuS)
cimetropium (Cim)
ipratropium (Ipra)
N-methyl-scopolamine (MeS)
CH
3
+
+
N
H
3
C
N
S
O
OH
OH
O
O
S
O
O
tiotropium (Tio)
trospium (Tros)
Fig. 1
Chemical structures of selected tropane alkaloids
plant secondary metabolites are hyoscyamine (atropine), scopolamine (hyoscine)
and cocaine, the latter one originated from
Erythroxylaceae coca
(Fig.
1
).
The following section will discuss chemical structures of TA and the resulting
implications for the design of analytical approaches.