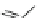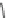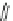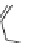Biomedical Engineering Reference
In-Depth Information
Indoles:
Others:
XXI) Molindone (276.2)
XXII) Loxapine (327.1)
C
16
H
24
N
2
O
2
C
18
H
18
ClN
3
O
N
H
O
N
N
N
Cl
O
O
Fig. 1
(continued)
cerebral areas such as the striatum, which is associated with motor control, has
been shown to lead to serious side effects.
Patients treated with typical APs are likely to suffer parkinsonian symptoms such
as mobility difficulties (tremor, bradykinesia, postural instability). The blockage of
DA receptors leads to a decrease in DA, ultimately causing symptoms consistent
with patients suffering from Parkinson's disease.
The use of typical APs is associated with a number of side effects which can
outweigh their positive outcomes at times. Neuroleptic Malignant Syndrome (NMS),
although difficult to distinguish from other disorders [
4
], is characterized by ele-
vated temperature, changed mental status, and severe muscle rigidity. While recent
studies suggest that the prevalence of NMS has decreased from around 2.4 % [
5
] to
0.01-0.02 % [
6
], this is most likely due to more conservative prescription patterns
of typical APs and a greater awareness of the illness [
7
]. The mortality rate associ-
ated with NMS was reported to be as high as 20 % at one stage [
5,
8
]. However, in
the last two decades, mortality rates have fallen below 10 % due to early recognition
and improved management [
6
] .
Additional problems associated with typical APs include cardiotoxicity [
9
] ,
seizures, and an increased risk of sudden cardiac death [
10,
11
] .
1.3
Atypical APs
Due to the broad range of side effects associated with typical APs, combined
with their inability to improve all symptoms of psychotic disorders, a new gen-
eration of APs was introduced in the 1970s. These APs are generally referred to
as “second generation” or atypical APs. This group includes indoles (e.g., zip-
rasidone and sertindole (Fig.
2
I-II)), benzamides (e.g., amisulpride, sulpiride
(Fig.
2
III-IV)) diazepines/oxazepines/thiazepines (e.g., clozapine, olanzapine,
quetiapine (Fig.
2
V-VII)), and others (e.g., aripiprazole, risperidone, buspirone,
paliperidone, zotepine (Fig.
2
VIII-XII)).
Clozapine, a tricyclic dibenzodiazepine derivative, was the first atypical AP to be
approved by the FDA in 1989. It was originally thought that increased affinity to the
5HT
2A
receptor, in combination with a lower or no affinity to the D
2
receptors, might
define a compound as an “atypical” AP [
12,
13
]. However, studies have shown that