Biomedical Engineering Reference
In-Depth Information
SWITCH
to SPE elution
SWITCH
to SPE load
40
30
20
10
Blank control blood
Drug spiked blood
0
0 2 4 6 8 0 2 4 6 8 0 2 4 6
Time [sec]
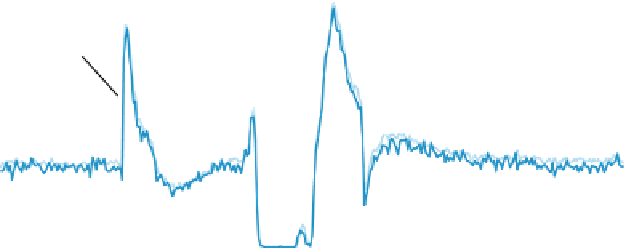
Fig. 1
Using a T-piece between HPLC and MS, a syringe pump can be utilized to infuse a constant
flow of analytes at therapeutic concentrations whilst drug spiked whole blood samples are delivered
via SPE-HPLC. Ion traces of the analytes (here everolimus at 6 ng/ml) are recorded. In this particu-
lar case, no ion yield attenuation due to the spiked drug can be observed, although strong effects can
be seen in the solvent front elution zone shortly prior to the analyte elution time window
often associated with longer periods of ion suppression to be associated with remain-
ing low-molecular weight matrix constituents.
Ion suppression is so far mainly considered in the context of sensitivity and the
lower limit of quantification of an assay. But it has to be emphasized that short term
variations in ion yields—particularly due to matrix components—can compromise
the accuracy of analyses: Whenever the variation of ion yield has a differential
impact on target analyte and internal standard, accuracy is compromised. This
means that the reliability of LC-MS/MS analyses critically depends on (1) how
similar the impact of ion suppression or ion enhancement on target analyte and
internal standard compound is and on (2) how similar the matrices of calibrator
samples and actual patients' samples are with respect to the modulation of ioniza-
tion efficacy. This problem can be of relevance for an entire measuring series—if
systematic differences in the ionization modulation properties of calibration materi-
als and actual patients' samples are present—or it may non-systematically affect
individual patients' samples as well.
In general, accuracy of LC-MS/MS analyses will be good if the physicochemi-
cal properties of target analyte and internal standard compound are very similar.
Stable isotope labelled compounds are ideal internal standards since they have
almost identical overall physico-chemical properties compared with their unla-
beled counterpart—the analyte. In MS these two species can easily be distinguished
by their molecular weight. In labelled compounds typically
1
H (hydrogen) is
exchanged by
2
H (deuterium) or carbon
12
C is exchanged by
13
C in several positions
of the molecule (ideally more than three atoms exchanged). It has to be stressed
that the physico-chemical behaviour of labelled and unlabelled compound is
not
completely
identical; the term isotope effects refers to these minor differences.