Biomedical Engineering Reference
In-Depth Information
WRM-1 nuclear export is not known. The requirements of kinesin for
WRM-1 localization raised the model that microtubule-dependent
transport of WRM-1 toward the cell cortex removes it from the
perinuclear region, enhancing its nuclear export (
Sugioka et al., 2011
).
3. ASYMMETRIC REGULATION OF TRANSCRIPTION
IN NUCLEI
In other organisms,
b
-catenin functions as a coactivator of transcription
factors of the TCF/LEF family. However, WRM-1 in
C. elegans
has a weak, if
any, activity to enhance transcription by POP-1/TCF (
Korswagen et al.,
2000; Natarajan et al., 2001
). Instead, WRM-1 in the nucleus regulates
nuclear export of POP-1 through LIT-1 kinase that is homologous to
Nemo-like kinase in mammals (
Lo, Gay, Odom, Shi, & Lin, 2004
)
(
Fig. 3.2
). WRM-1 can bind to both LIT-1 and POP-1 (
Rocheleau et al.,
1999; Yang et al., 2011
). Upon WRM-1 binding, LIT-1 is activated to
phosphorylate POP-1. Phosphorylated POP-1 binds to PAR-5/14-3-3
and is exported out of nucleus (
Lo et al., 2004
). Similar to WRM-1, LIT-
1 is also enriched to the posterior nucleus after asymmetric divisions,
probably through its tight binding to WRM-1 (
Lo et al., 2004; Takeshita
& Sawa, 2005
). Therefore, the phosphorylation and nuclear export of
POP-1 occurs asymmetrically in the posterior nucleus, creating high/low
POP-1 asymmetry (higher in the anterior nucleus) that is opposite to the
nuclear low/high WRM-1 asymmetry (higher in the posterior nucleus)
(
Fig. 3.1
C) (
Lin, Thompson, & Priess, 1995
).
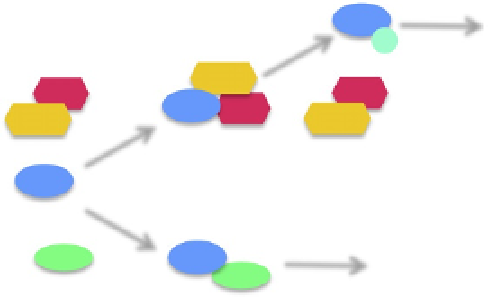
Nuclear
export
POP
P
WRM
LIT
WRM
LIT
POP
LIT
WRM
POP
SYS
POP
Transcription
activation
SYS
Figure 3.2 Regulation of transcription by POP-1. Free POP-1 binds either to SYS-1 to
activate transcription or to the WRM-1/LIT-1 complex to be exported out of nuclei.