Biomedical Engineering Reference
In-Depth Information
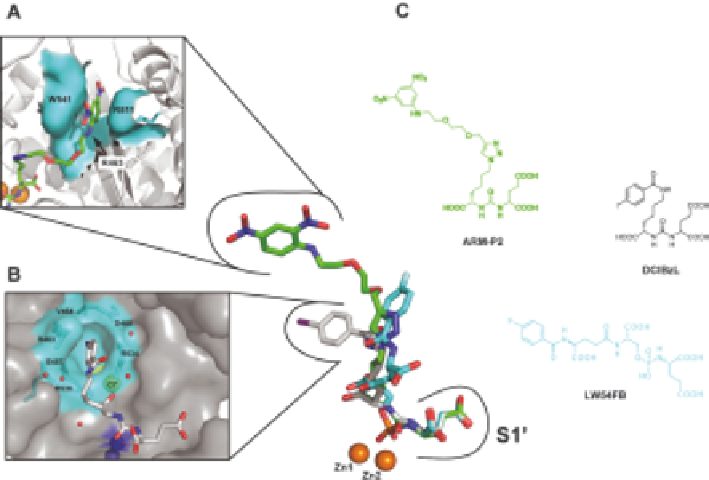
Figure 3.4
P1 diversified inhibitors and the distal binding sites. Superposition of three
NAAG-based inhibitors (shown in stick representation) ARM-P2 (green;
PDB code 2XEI), DCIBzL (gray; PDB code 3D7H), and LW54FB (cyan;
Barinka et al., unpublished). While the positioning of the P1
0
glutamate
moieties is almost indistinguishable, diversified distal parts (D-parts)
assume different positions in the extended S1 pocket. Panels (A) and (B)
offer a detailed view of the structural arrangements of the arene-binding
site (A) and the S1 accessory hydrophobic pocket (B). (C) Molecular
formulae of the three inhibitors.
GCPII inhibition is neuroprotective during glutamate-mediated excitotoxi-
city in two ways. First, it stops glutamate release from NAAG in extracellular
space. Second, it increases levels of NAAG. NAAG itself acts as an agonist of
group II mGluRs. Activation of this subgroup of metabotropic receptors
during excessive neuronal activation contributes to the prevention of pre-
synaptic release of glutamate and GABA.
11,114
Acting on glial cells, NAAG
also helps to stimulate the release of neuroprotective growth factors.
115
This mechanism of action is utilized in pre-clinical experimental treatment of
both acute and chronic neurological disorders related to excess glutamatergic
transmission (see recent reviews
15,116-118
). Both NAAG and GCPII are
expressed in spinal cord, where NAAG levels can affect glutamatergic trans-
mission of pain perception, which involves activation of group II mGlu
receptors on sensory neurons.
7,8,119
GCPII inhibitors have been proposed as
potential analgesic drugs and have decreased both inflammatory and neuro-
pathic pain in rats.
92,99,120,121
A study by Ghadge et al. supported the
hypothesis that NAAG is involved in regulation of glutamate levels in spinal
cord and showed that GCPII inhibition can protect motor neurons in a mouse