Biomedical Engineering Reference
In-Depth Information
A
B
C
D
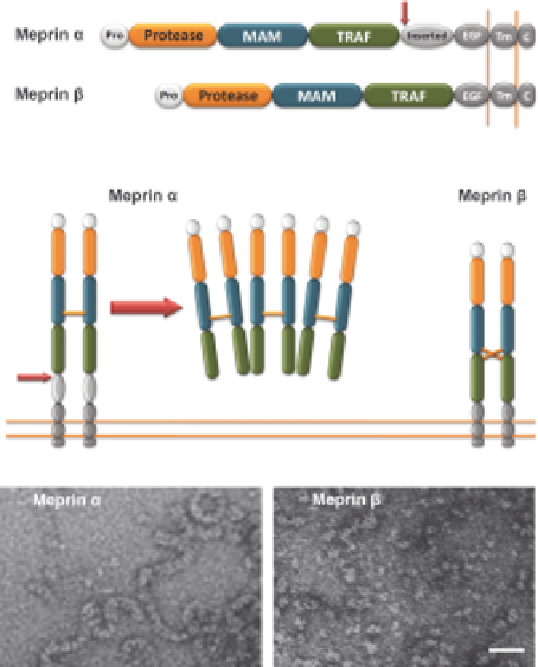
Figure 2.2
Domain composition and quaternary structure of meprin a and b. Both
meprins are initially expressed as membrane-bound pro-enzymes (A).
They both comprise a pro-peptide (Pro),
31
an 'astacin-like' protease
domain (Protease),
36
a 'meprin A5 protein tyrosine phosphatase m-like'
domain (MAM), (Aricescu et al. 2006) a 'tumor necrosis factor a receptor
associated factor-like' domain (TRAF), (Ni et al. 2000) an 'epidermal
growth factor-like' domain (EGF), a transmembrane domain region (TM)
for attachment to the plasma membrane, and a cytoplasmic tail (C).
17
Additionally, meprin a contains an inserted domain that is proteolytically
cleaved (red arrow) in the secretory pathway, subsequently leading to
secretion of the enzyme (A, B). Both meprins form disulfide-linked dimers
via inter-subunit disulfide bridges in the MAM-TRAF region (B). Meprin
a tends to form huge oligomeric complexes, thus revealing this protein as
the largest secreted protease known. This is reflected in electron-micro-
scopy pictures of recombinant human meprins, exhibiting ring- and chain-
like structures for meprin a (C), whereas meprin b appears as small
globular particles (D). Bar: 20 nm.
residues in P1
0
(nomenclature by Schechter and Berger), while meprin a rather
favors small aliphatic residues.
17,42
Indeed, proteomics has revealed an excep-
tionally high preference for aspartate and glutamate for both human meprin a