Biomedical Engineering Reference
In-Depth Information
expressed protein. The fusion and tags are removed during self-catalyzed activa-
tion by cleavage seven amino acids N-terminal to the cleavage site observed in
the natural system.
19
The thioredoxin fusion keeps the protein soluble and
facilitates isolation. The resulting mature plasmepsin I was used by Bhaumik
et al.
26
to obtain crystals of the apoenzyme as well as inhibitor-bound complexes.
The three-dimensional structure of plasmepsin I, like the other plasmepsins
that will be described in the following sections, is strongly related to the classic
archetype enzyme of the aspartic peptidase class, pepsin from the stomach of
animals. Initially, porcine pepsin was studied because it was available in large
quantities from digestive juice of pigs and could also be produced from the
precursor protein, pepsinogen, which could be isolated from the cells that
line the stomach cavity. Based on extensive crystallographic studies cataloged
in the Protein Databank (http://www.rcsb.org), enzymes in this family have a
bilobal domain structure where the N-terminal and the C-terminal domain are
of nearly equal length (ca. 165-170 amino acids) and a very similar beta sheet
structure. In fact, each domain has two orthogonally arranged beta sheets
that form the 'body' of the domain. The various connecting loops between
the segments of beta strands making up the two sheets provide some of the
amino acid residues that are able to interact with substrates and inhibitors that
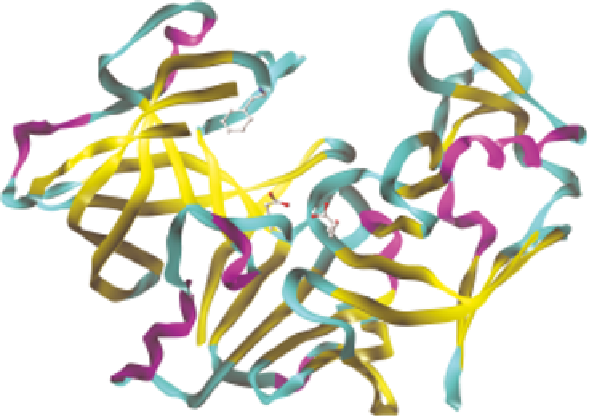
bind into the active site cavity that lies between the two domains. Figure 11.1
shows several views of the plasmepsin I structure to illustrate these points.
Figure 11.1
Overall structure of P. falciparum plasmepsin I (PDB: 3QVR, Bhaumik
et al.
26
). The extensive beta strand structure can be seen in both halves of
the molecule, with the N-terminal domain on the left hand side of the
figure and the C-terminal domain on the right hand side. The orthogonal
nature of the two beta sheets in each domain can best be seen in the N-
terminal half in this view of the structure. The two catalytic aspartic acids
can be seen at the bottom of the deep active site cleft.