Biomedical Engineering Reference
In-Depth Information
D
SMIPP-S-D1
Y200
T145
Q192*
N143*
N
V197
A
180˚
A142
V191
L107
P13
Q
I105
K103
I228
L31
SS1
D98
L97
123456789
Average
Variable
Conserved
Insufficient data
E
SS2
SS3
Y200
A
180˚
N/D
SS1
Q/H
Figure 10.4
Continued.
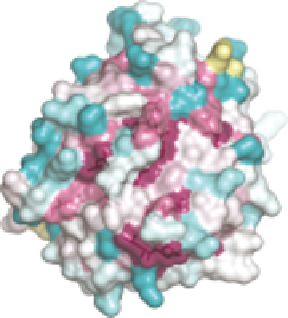
SMIPPs (Figure 10.4E); regions of lower sequence conservation show the lar-
gest structural shifts. Interestingly, there are small patches of conserved regions
on the opposite face of the molecule to the active site (Figure 10.4C-E). These
are in regions of good agreement between the SMIPP structures, suggesting
that they may represent potential binding sites or exosites. All but one of the
predicted N-glycosylation sites for both SMIPPs lie within conserved regions
on this face, and this may provide a plausible explanation for the conservation
at these sites (Figure 10.4C and D).