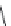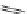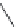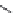Biomedical Engineering Reference
In-Depth Information
stability in plasma compared to the original compounds. Despite the fact that
KLK3/PSA can cleave activating peptides when at high concentrations, the
modified peptides exhibited a higher stability.
85
9.2.4 Depsipeptides
Depsipeptides are peptides that carry at least one ester linkage in place of
amide.
86
A variety of natural sources produce depsipeptides, including fungi,
bacteria, and various marine organisms. A class of depsipeptides (General
Formula 9.4) isolated from strains of chondromyces bacteria or modified from
the original peptides isolated by simple chemical procedures were found to
specifically inhibit KLK7.
87
Compounds 9.5, 9.6, 9.7, and 9.8 have an IC
50
of
1000, 400, 700, and 200 pM, respectively, while they are 410-fold more specific
for KLK7 than for human neutrophile elastase, which is also a chymotrypsin-
like protease. Compounds 9.5, 9.6, and 9.7 were extracted from Chondromyces
crocatus, while compound 9.8 was derived from a chemical reaction of 9.5 with
3-(chloromethyl)-1,5-dimethyl-1H-pyrazole.
Compound 9.5 has been assessed in experimental animals for the treatment
of skin diseases. Specifically, in the mouse skin barrier disruption model
(repeated skin stripping by S-Sqame skin sampling disks), it was found to
accelerate barrier repair by 57%. Also, a single application of a solution of 9.5
in an oxazolone model of allergic contact dermatitis (ACD) in mice resulted in
40% inhibition of inflammatory ear swelling at 10 mM, while at 30 mM, it
caused 46% inhibition of inflammation. Finally, in the 2,4-dinitro-
fluorobenzene (DNFB) model of ACD in swine, two applications of 1%
solution of 9.5 inhibited inflammation by 30% and skin redness by 27%.
87
O
O
H
O
NH
N
O
O
OH
N
O
R
8
O
R
3
H
O
O
N
H
O
NH
O
N
H
N
R
5
O
OH
R
2
O
A
1
R
7
H
2
N
O
H
O
X
O
N
R
9
O
H
Y
R
8
=
R
9
=
O
R
6
9.5
9.6
H
H
(H
3
C)
2
HC
9.4
(H
3
CH
2
C)(H
2
C)HC
9.7
H
H
3
C
N
N
9.8
(H
3
C)
2
HC
H
3
C