Biomedical Engineering Reference
In-Depth Information
(3KQZ.pdb
42
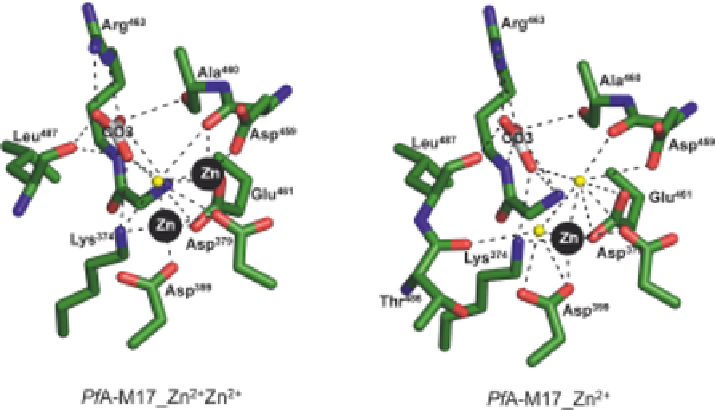
). The two metal ions are nearly 0.3 nm apart (Figure 7.5A). The
tight-binding site 1 zinc is coordinated by Asp
379
, Asp
459
, and Glu
461
, while
coordination of the catalytic site 2 zinc is coordinated by Lys
374
, Asp
379
,
Asp
399
, and Glu
461
, and a single water molecule, creating a hexameric coor-
dination of this ion (Figure 7.5A). The active site of PfA-M17 also contains a
carbonate ion that is not added to LAP preparations or crystallization
experiments but is always found within the active site.
44
The carbonate ion is
well coordinated, forming hydrogen bonds to residues Lys
374
, Ala
460
, Gly
462
,
Arg
463
, and Leu
487
(Figure 7.5). The carbonate ion has been proposed to act
as a general base, accepting the proton and activating a (nucleophilic) metal-
bridging water.
44
Resolution of the X-ray crystal structures of PfA-M17 also demonstrated
that the 'loosely' bound zinc does not contribute to the overall stability of the
active site. A 0.2 nm X-ray crystal structure PfA-M17_Zn
21
(obtained from
purified protein that lacked supplementary zinc ions) clearly showed a vacant
position at site 1. The position of the modeled and refined zinc ion in the tight
bound site 2 had slightly shifted by
0.04 nm, and high B-factors indicated
that this ion was subject to local disorder (or movement). The bound zinc was
coordinated by two water molecules to complete the hexameric coordination
(Figure 7.5B).
B
A
B
Figure 7.5
Diagram of metal cation coordination in active site of PfA-M17 where (A)
both catalytic sites are occupied, and (B) only 'tight' binding site 2 is
occupied. Carbon atoms of residues are colored green, zinc is shown as
black spheres, water molecules are shown as light-yellow spheres, and the
carbon atoms of CO3 ion are colored gray. Hydrogen and metallo-bonds
are indicated (dashed lines).