Biomedical Engineering Reference
In-Depth Information
FEED
RETENTATE
PERMEATE
FIGURE 14.7
Filtration—selective mass transfer by means of pore size and size of substances compared to pore
size.
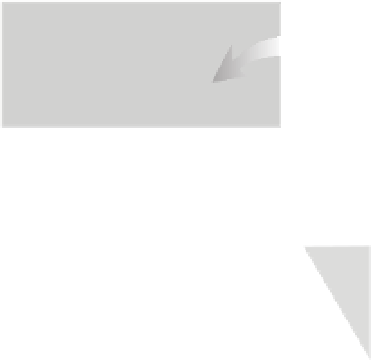
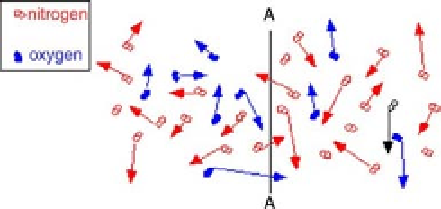
FIGURE 14.8
Random movement of particles resulting in a net movement across a dividing line based on
relative concentrations on both sides of the line.
From Time Domain CVD.
Again, the net movement of any substance (within a mixture of substances as just shown)
across line A-A is a function of the concentration of that substance on each side of the line. It
is certainly possible that the net movement of one substance is in the opposite direction of
another, each based on its own concentration gradient. This is also true for mass transfer
across pores. The concentration gradients control the amount and the direction of mass
transfer for each substance. When a substance, such as an ion, is in a solution (such as
within extracellular fluid), then the substance is known as a
solute
and the fluid as a
solvent
.
Mass transfer of water is known as
osmosis.
Further, the relative size of the solute as compared to the pore size may affect the poten-
tial mass transfer by either (1) completely restricting the movement (if the species is larger
than the pore size) or (2) merely restricting it because it may be nearly equal to the pore
size. In such cases, Fick's Law can be modified to account not only for the limited pore area
(via the permeability) but also via the pore to species size, which is given by the
restrictive
diffusivity (D
R
). Thus, Fick's Law could be rewritten as
Mass exchange rate
dx
A typical cell membrane is thin (80-100
˚
thick) and elastic. Membranes typically consist
of 60 to 65 percent proteins, 30 to 35 percent lipids, and 5 to 10 percent polysaccharides and
¼
D
R
A
P
dC
=