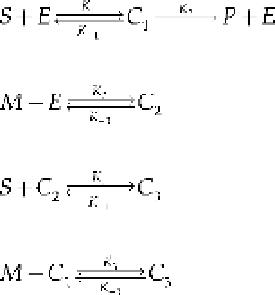Biomedical Engineering Reference
In-Depth Information
þ
K
K
s
M
¼
K
1
2
K
1
K
i
M
¼
K
4
þ
K
5
K
4
K
i
3
¼
K
3
K
3
K
i
6
¼
K
6
K
6
The reaction rate is
V
¼
q
P
¼
K
q
C
1
þ
K
q
C
3
2
5
8
<
9
=
2
0
@
1
A
þ
K
i
6
q
M
3
0
K
K
3
K
i
6
q
S
þ
K
i
3
K
K
4
K
i
6
þ
K
1
1
1
4
K
6
K
i
M
5
K
E
2
:
;
ð
8
:
129
Þ
q
S
8
<
9
=
K
1
K
3
q
S
þ
K
1
K
4
K
i
3
þ
K
1
K
6
K
s
M
þ
q
M
þ
K
5
E
0
q
M
:
;
¼
D
This reaction rate is quite complex compared with the others derived previously. The reac-
tion velocity from Eq. (8.129) is plotted in Figure 8.28. As observed, as the quantity of the
allosteric modifier increases, it reduces
max
and the reaction rate.
Reaction Rate from the True Steady-State
We will consider a simpler allosteric modifier model
7
to further investigate the velocity
of the reaction using the true steady-state rather than the quasi-steady-state approximation
in the analysis. Here, the product is synthesized only from the intermediate complex
V
C
1
,
and a single reaction rate is used for
S
and
M
as follows:
ð
8
:
130
Þ
7
This model is based on Keener and Sneyd, page 11.