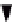Biomedical Engineering Reference
In-Depth Information
f
1
(t)
f
2
(t)
K
12
V
max
q
1
(
q
1
+
K
M
)
q
1
q
2
K
21
K
20
q
2
K
10
FIGURE 8.14
A two-compartment model with a quasi-steady-state approximation and biochemical reaction
transfer rates. In addition, a constant input from compartment 1 into compartment 2 is used, with
K
12
q
1
as the transfer
rate.
Consider the model shown in Figure 8.14 that contains a quasi-steady-state approxima-
tion and a biochemical reaction as transfer rates. We also have a constant transfer of sub-
strate from compartment 1 into compartment 2 by using
K
12
q
1
as the transfer rate. The
equations that describe this system are
0
1
V
@
max
q
1
þ
K
M
A
q
1
þ
K
21
q
2
þ
f
1
ð
t
Þ
q
1
¼
K
12
K
10
þ
ð
Þ
ð
8
:
67
Þ
2
2
q
2
¼
K
12
K
20
q
þ
f
2
ð
t
Þ
Note that the constant loss from compartment 1 and input to compartment 2 could describe
an active pump. Also note that a chemical reaction in compartment 2 consumes the sub-
strate with rate
K
20
. We will expand on these concepts with multicompartment models in
the next section that includes diffusion.
8.4 DIFFUSION, BIOCHEMICAL REACTIONS,
AND ENZYME KINETICS
In Chapter 7, we discussed diffusion as a flow of ions down the concentration gradient.
Up to now, we have approached biochemical reactions and enzyme kinetics occurring in a
homogeneous volume. Now, we include diffusion from another compartment, biochemical
reactions, and enzyme kinetics in our analysis. As we will see, the movement of a substrate
or an enzyme into the cell by diffusion allows a product to be created. This product then
can be used inside the cell or diffused out of the cell to be used by another cell or tissue.
Additionally, the same situation occurs within the organelles of the cell. These reactions
can serve a regulatory role as well as accelerating biochemical reactions; recall the reaction
involving ADP and ATP in the mitochondria, where the availability of ADP regulates the
production of ATP.