Biomedical Engineering Reference
In-Depth Information
q
B
(Approx.)
10
10
8
8
6
6
q
B
(True)
4
4
2
2
0
0
0
0.04
0.08
0.12
0.16
0.2
0
1
2
3
4
5
Time
Time
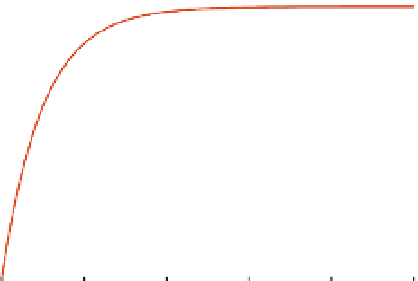
FIGURE 8.4
A rate limiting sequential reaction for
K
2
. Note that both the approximation and true solution for
q
P
are drawn in the figure on the right.
Þ
e
K
2
t
:
Þ
K
2
e
K
2
t
, which
q
B
¼
K
2
q
B
, which has the solution
q
B
¼
q
A
ð
0
Next,
q
P
¼
K
2
q
B
¼
q
A
ð
0
:
e
K
2
t
has the solution
q
P
¼
q
A
ð
0
Þ
1
While quasi-steady-state assumes that reactant
A
is
immediately in steady state at time 0 and reactant
B
rises to
q
A
ð
0
Þ
immediately, there is a
, in which
5
K
period of time,
rises.
Figure 8.4 illustrates the approximation in Eq. (8.31), with the true solution in Eq. (8.29)
given with
q
A
falls to 0 and
q
B
1
q
A
ð
0
Þ¼
10,
q
B
ð
0
Þ¼
0and
q
P
ð
0
Þ¼
0,
K
¼
500, and
K
¼
2
:
For
q
B
, the approxima-
1
2
5
tion is quite accurate after it reaches quasi-steady-state,
t
¼
K
1
¼
0
:
01
:
For
q
P
, the approxi-
mation is quite accurate the entire duration.
8.2 ENZYME KINETICS
As described earlier, catalysts are substances that accelerate reactions but are not con-
sumed or changed by the reaction. Most chemical reactions in the body can occur without
the presence of catalysts, but they occur at a very low rate. An enzyme is a large protein that
catalyzes biochemical reactions in the body. These reactions convert a reactant, now called a
substrate because it involves an enzyme, into a product by lowering the free energy of acti-
vation. Enzymes can increase the rate of the reaction by an order of thousands to trillions.
Each enzyme is highly specific and only allows a particular substrate to bind to its active
site. Many enzymes are used in the control and regulation of functions of the body.
In general, an enzyme reaction involves a series of reactions. Binding the enzyme with
the substrate is the first step in creating an intermediate complex, which increases the abil-
ity of the substrate to react with other molecules. The next step is when the substrate-
enzyme complex breaks down to form the free enzyme and product. Both the first step
and the second step are reversible, but in the second step, the reverse reaction is so small
that it is often omitted.