Biomedical Engineering Reference
In-Depth Information
With this insight into the elementary structure of atoms, Rutherford was the first to
recognize that radioactive emissions involve the spontaneous disintegration of atoms. After
the general acceptance of this basic concept, Rutherford was awarded a Nobel Prize in
chemistry in 1908, and the “mystery” began to unfold. By observing the behavior of these
radioactive emissions in a magnetic field, the Curies discovered that there are three distinct
types of active radiation emitted from radioactive material. The three, arbitrarily called
alpha, beta,
) particles, which
are positively charged and identical to the nucleus of the helium atom; (2) beta (
and
gamma
by Rutherford, are now known to be (1) alpha (
a
b
) particles,
which are negatively charged electrons; and (3) gamma (
g
) rays, which are pure electromag-
netic radiation with zero mass and charge.
15.2.2 Elementary Particles
By the 1930s, the research in this field clearly identified three elementary particles: the
electron, the proton, and the neutron, usually considered the building blocks of atoms.
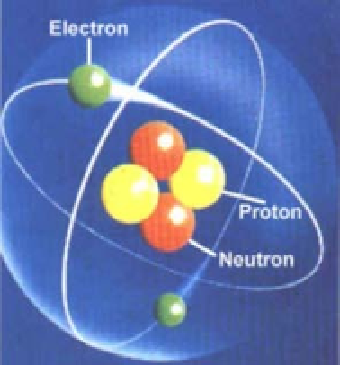
Consider the arrangement of these particles as shown in Figure 15.3, a common view of
the atom. The atom includes a number of particles: (1) one or more electrons, each having
a mass of about 9.1
10
31
kg and a negative electrical charge of 1.6
10
19
coulombs;
10
27
kg, which is approximately 1,800 times
that of the electron; and (3) perhaps neutrons, which have the same mass as protons but
possess no charge.
Neutrons and protons exist together in the nucleus and have been given the collective
name of
(2) at least one proton with a mass of 1.6
. The total number of nucleons in the nucleus of an element is called the
atomic mass, or mass number, and is represented by the symbol (A), whereas the number
of protons alone is referred to as the atomic number (Z). Atoms having the same A, such
as Carbon-12 and Boron-12, are called isobars. Atoms with the same number of neutrons
are called isotones.
nucleons
FIGURE 15.3
A common view of the atom.
Compliments of http://www.eskom.co.za/nuclear_energy/fuel/atom.jpg.