Biomedical Engineering Reference
In-Depth Information
Pulsed sensor
Potentiometric sensor
1.7
2.0
1.3
1.7
2.0
20 mV
20 mV
1.3
1.0
1.0
0.7
0.3
0.0
0.3
0.7
0.3
0.0
0.3
0
10
20
30
40
0 0 0 0 0
Time (min)
Time (min)
(a)
(b)
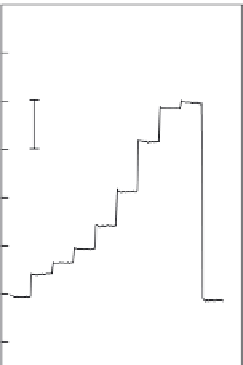
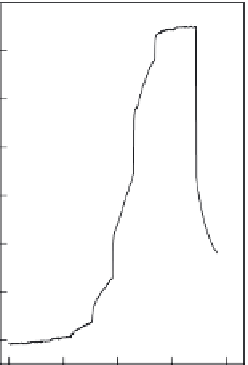
FIGURE 4.11
Amplitude-time behavior of potential during calibration in 0.1 M NaCl solution contain-
ing 50 mM TRIS (pH 7.40) with a pulsed galvanostatic sensor (a) and a classical potentiometric protamine-
selective electrode (b) [54]. Logarithmic protamine concentrations (mg l
1
) are indicated on the traces. The
fi rst and last samples did not contain protamine.
applied current gives an additional degree of freedom. The tunability of the selectivity
by the magnitude and sign of the applied current and the capability of such sensors to
monitor more than one analyte at the same time was demonstrated [51]. Electrochemical
control of ion fl uxes allows one to detect simultaneously with a single sensor the cal-
cium activity and total concentration of complexed calcium [56].
Perhaps one of the most intriguing applications of this methodology is the detection
of ions within the so-called super-Nernstian response region in a defi ned range of ion
activity [55]. The response function of a Ca-selective galvanostatic sensor has a near-
Nernstian response slope at relatively high activities of calcium. Indeed, at lower cal-
cium activities a drastic potential change occurs with a slope much higher than Nernstian
(Fig. 4.12a). The origin of this behavior lies in a concentration polarization at the phase
boundary under an applied constant current pulse, which is caused by the depletion of
the aqueous diffusion layer adjacent to the membrane [51]. There is a complete analogy
with similar responses of traditional ISEs where the super-Nernstian behavior is due
to the uptake of primary ions into membrane phase [57]. However, in traditional ISEs
there are a variety of parameters that may infl uence this response function, including
the activities of interfering ions of the sample and inner solution, the concentrations of
ionophore and lipophilic ion exchanger in the membrane, the thickness of the Nernstian
diffusion layers and the diffusion coeffi cients in both phases [58]. As full control over
these parameters is not possible, a poor reproducibility and signifi cant drift of the sensor
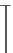









Search WWH ::

Custom Search