Biomedical Engineering Reference
In-Depth Information
6
4
2
0
2
0
0.05
0.1
0.15
0.2
NaCl + PA
0.25
NaCl
0
1
11
12
22
23
Time (s)
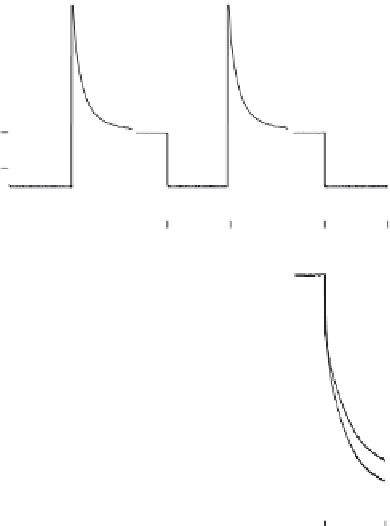
FIGURE 4.10
Current-time traces (top) and potential-time traces (bottom) for the pulsed galvanostatic
measurement of 10 mg l
1
protamine in aqueous sample containing 0.1 M NaCl and 50 mM TRIS (pH 7.40)
[54]. An applied cathodic current of
3 µA leads to the extraction of protamine into the membrane, and the
observed potential is signifi cantly different for samples with and without protamine (bottom). The mem-
brane is renewed potentiostatically at 0 V for 10 sec before the next current pulse.
The behavior of potentiometric and pulsed galvanostatic polyion sensors can be
directly compared. Figure 4.11 shows the time trace for the resulting protamine cali-
bration curve in 0.1 M NaCl, obtained with this method (a) and with a potentiometric
protamine membrane electrode (b) analogous to that described in [42, 43]. Because of
the effective renewal of the electrode surface between measuring pulses, the polyion
response in (a) is free of any potential drift, and the signal fully returns to baseline
after the calibration run. In contrast, the response of the potentiometric protamine elec-
trode (b) exhibits very strong potential drifts.
Galvanostatically pulsed sensors can be employed for heparin determination via
titration with protamine using protocol described earlier [42, 43] and initial experi-
ments showed that heparin detection in whole blood samples can be accomplished with
this technology.
This method can be applied not only for polyion detection but for the detection of
small ions as well. In contrast to potentiometric electrodes the external control of the
























Search WWH ::

Custom Search