Biomedical Engineering Reference
In-Depth Information
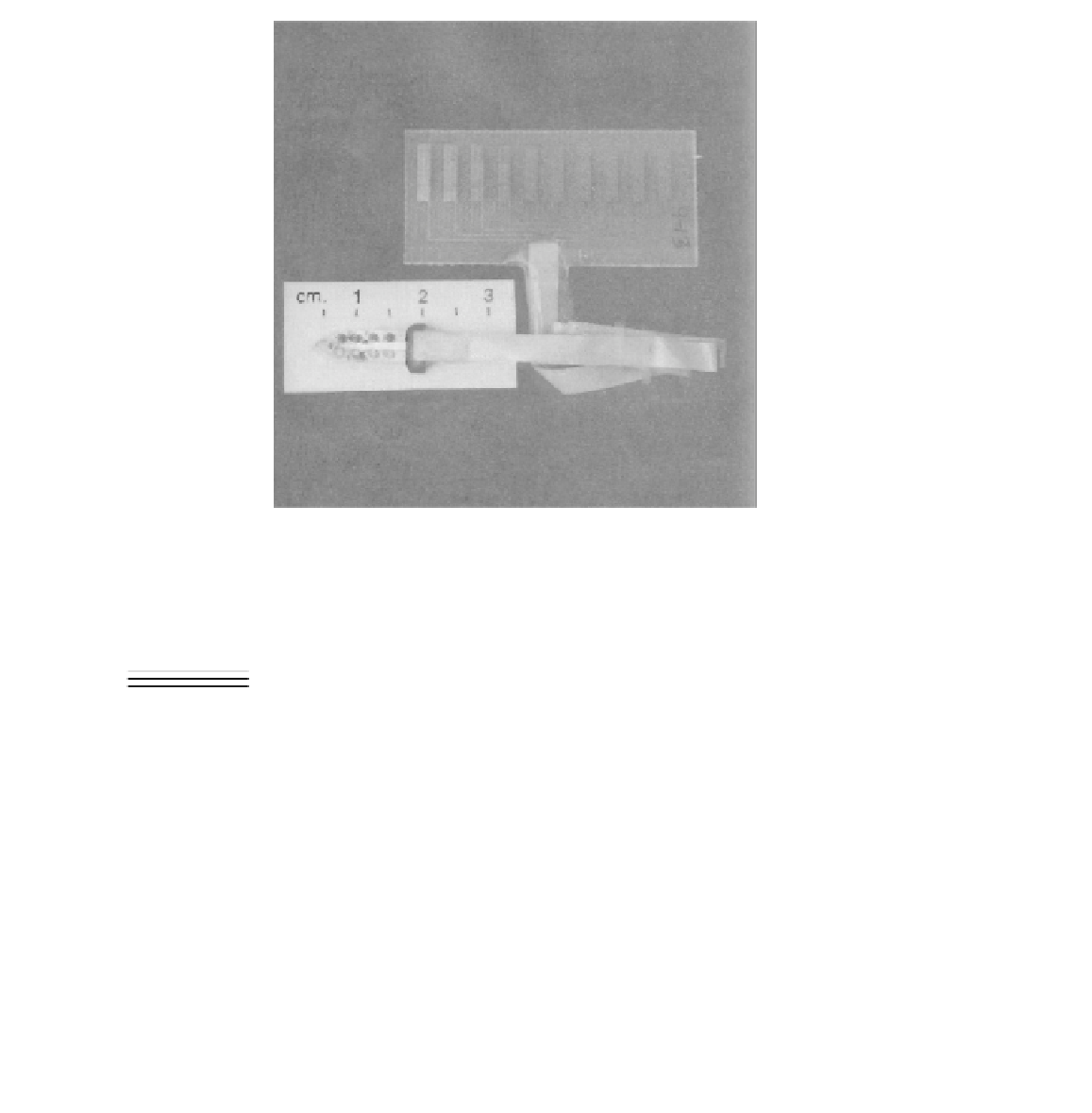
FIGURE 4.3
Miniaturized planar nine-site H
/K
selective sensor array applied to
in-vivo
measure-
ments in porcine cardiovascular system. (From [107].)
4.2 CLASSICAL ION-SELECTIVE ELECTRODES
4.2.1 Understanding of the operational principles
An established setup for measurements with ion-selective electrodes is depicted in
Fig. 4.4. An ion-sensitive membrane is placed between two aqueous phases, i.e. a sam-
ple and an internal fi lling solution. A reference Ag/AgCl electrode is placed into the
inner fi lling solution, which contains ions this electrode is responsive to. The external
reference electrode is placed in the sample and usually has a salt bridge in order to
prevent sample contamination. The membrane is an essential part of the sensor and is
made of glass (oxide or chalcogenide), crystalline material (monocrystal or polycrys-
talline) or water-immiscible liquids (highly plasticized polymers, solvent impregnated
porous fi lms, etc.).
Potentiometric measurements are usually performed under zero-current conditions
in a galvanic cell of the type:
Ag | AgCl | KCl
sat
| 3 M KCl || sample solution || membrane || inner fi lling solution | AgCl | Ag




Search WWH ::

Custom Search