Biomedical Engineering Reference
In-Depth Information
electrode of
580 mV and was partially reduced in constrast to the microsomal CYP
which was not detectable. Besides the mercury electrode, the solid electrodes are
widely used to study the electrochemical behavior of CYP. But the rather low electron
transfer between the protein and the electrode at bare solid electrodes limits their use
for the construction of effi cient CYP biosensors. Thus, electrodes modifi ed with com-
pounds that can facilitate electron transfer, prevent denaturation of protein, and cause
appropriate orientation of the protein have been widely used.
A biosensor based on mediator-free CYP2B4 catalysis by immobilizing monomer-
ized CYP2B4 in montmorillonite was studied by Shumyantseva [222]. When substrates
were added to air saturated buffer solution, there was an increase in the reduction cur-
rent. A typical concentration dependence measured in chronamperometry is shown for
aminopyrine and benzphetamine (Fig. 17.4). The reaction was inhibited by metyrap-
one. This indicates the catalytic activity of CYP2B4 in the presence of substrate.
The majority of CYP enzymes are located in a hydrophobic environment in
the endoplasmic reticulum of cells, although cytosolic enzymes also exist, such as
CYP101. In order to mimic the physiological environment of CYP enzymes, a number
of groups have used phospholipids to construct biosensors such as DDAB, dimeristoyl-
L-
-phosphatidylcholine (DMPC), dilauroylphosphatidylethanolamine (DLPE) and
distearoylphosphatidylethanolamine (DSPE). Phospholipid layers form stable vesicu-
lar dispersions that bear structural relationship with the phospholipid components of
biologically important membranes. By this way a membranous environment is created
that facilitates electron transfer between the enzyme's redox center and the electrode.
A CYP101 biosensor was created for monitoring drug conversion by this approach
[223]. The biosensor comprised a GC electrode modifi ed with CYP101 contained in
DDAB vesicle dispersion. CVs of the CYP electrode in air-free buffer showed direct
electron exchange between the heme group of CYP101 and the electrode. The
E
0
was found to be
α
10 mV at a scan rate of 500 mV s
1
and the peak separa-
tion was 36 mV. When 3 mM of ethanolic solution of camphor was added to the meas-
uring buffer, a catalytic response was observed. The cathodic peak potential and peak
260
1.5
1.2
0.9
0.6
0
3
6
9
12
[Aminopyrline], mM
FIGURE 17.4
Relationship of catalytic current, obtained with CYP2B4 biosensor after addition of ami-
nopyrine, with increasing concentrations of aminopyrine. (From [222], with permission.)


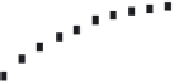






Search WWH ::

Custom Search