Biomedical Engineering Reference
In-Depth Information
[7, 22]. The hydrolysis of metal alkoxide (e.g. TEOS or TMOS) precursors results in
the formation of silanol groups (Si-OH), through condensation these silanol moieties
react further and form siloxanes (
ß
Si-O-Si
ß
), fi nally through polycondensation of
silanol and siloxanes SiO
2
matrices are formed after aging and drying processes as
shown in Eqs (1)-(3) (Fig. 16.1) and Fig. 16.2. The resulting sol-gel is an intercon-
nected rigid network with pores of submicrometer dimensions and polymeric chains
whose average length is greater than a micrometer. HCl and ammonia are the most
generally used catalysts for the hydrolysis, however, other catalysts such as acetic acid,
KOH, amines, KF, and HF are also used. The rate and extent of the hydrolysis are
mostly infl uenced by the strength and concentration of the acid or base catalyst [25].
When the liquid in the pore is removed at or near ambient pressure by thermal evapo-
ration, drying and shrinkage occurs, the resulting monolith is termed xerogel. If the
liquid is primarily alcohol, the monolith is termed alcogel.
In recent years, silica sol-gel-based inorganic-organic hybrid materials have also
been reported. The introduction of various functional groups into organic alkoxide has
led to organically modifi ed sol-gel glasses (ormosils). Some of the ormosil monomers
and ormosil formations can be found in Fig. 16.3. Redox molecules can be coupled
with the ormosil monomer functional group. The immobilized redox molecules can
(RO)
3
SiOR
H
2
O
(RO)
3
SiOH ROH
(1)
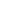
(2)
(3)
2(RO)
3
SiOH
(RO)
3
SiOH ROSi(OR)
3
(RO)
3
Si-O-Si (OR)
3
H
2
O
(RO)
3
Si-O-Si (OR)
3
ROH
FIGURE 16.1
Reaction scheme for formation of sol-gel.
TMOS/TEOS
sol-gel precursors
Enzyme or
biomolecules
Thin film on
electrode surface
Aging
Gel formation
Grinding
Sol-gel column
Entrapped biomolecules
FIGURE 16.2
Schematic diagram of sol-gel process.
























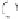




















































































































Search WWH ::

Custom Search