Biomedical Engineering Reference
In-Depth Information
PDDA/GC
CNT
GOx
Repeat
FIGURE 15.15
Schematic description of the fabrication of multilayer fi lms of GOx onto CNTs.
types of carbon electrodes. Zhao
et al.
have reported the direct electrochemistry of
HRP [105] and GOx [106] at carbon nanotube powder microelectrodes. Liu
et al.
have
probed the direct electron transfer between the redox active center of GOx, fl avin ade-
nine dinucleotide (FAD), and the CNT-modifi ed gold electrodes [107]. These results
are helpful in understanding the intrinsic redox behaviors of enzymes. Furthermore,
the immobilized GOx retains its bioelectrocatalytic activity for the oxidation of glu-
cose [72, 108], which can be used to fabricate a glucose biosensor.
Negatively charged species such as carboxylic acid group in acid-treated CNTs can
attract positively charged enzymes from solution as long as the pH value of the enzyme
solution is controlled to be lower than the iso-electric point of the enzyme; thus, mul-
tilayer fi lms of the enzyme can be formed by the layer-by-layer technique. For exam-
ple, fi ve layers of GOx can be immobilized on the electrode surface by alternatively
dipping a poly(diallyldimethylammonium chloride (PDDA))-functionalized GC into a
CNT solution and a GOx solution (pH 3.8). Figure 15.15 illustrates the preparation
process for the formation of a multilayer fi lm of GOx on the electrode.
The cyclic voltammograms of the GOx/CNT-modifi ed GC electrodes in phosphate
buffer solution (pH 7.4) show two pairs of redox peak currents. The fi rst pair of peaks
(
E
1/2
0.09 V vs Ag|AgCl) is attributed to the carboxylic acid groups in CNTs, while
the second pair of peak currents (
E
1/2
0.46 V vs Ag|AgCl) is assigned to the direct
electron tranfer of GOx. These peak currents increase as the number of layers increase
indicating that the effective immobilization of GOx on CNTs using the layer-by-layer
technique (Fig. 15.16, see Plate 17 for color version).
15.3.4 Electrochemical biosensors based on carbon nanotubes
CNTs offer an exciting possibility for developing ultrasensitive electrochemical
biosensors because of their unique electrical properties and biocompatible nanostruc-
tures. Luong
et al.
have fabricated a glucose biosensor based on the immobilization of
GOx on CNTs solubilized in 3-aminopropyltriethoxysilane (APTES). The as-prepared
CNT-based biosensor using a carbon fi ber has achieved a picoamperometric response
current with the response time of less than 5 s and a detection limit of 5-10
M [109].
When Nafi on is used to solubilize CNTs and combine with platinum nanoparticles, it
displays strong interactions with Pt nanoparticles to form a network that connects Pt
nanoparticles to the electrode surface. The Pt-CNT nanohybrid-based glucose biosensor
µ
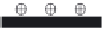































Search WWH ::

Custom Search