Biomedical Engineering Reference
In-Depth Information
DA
4
I
AA
2
a
0
0
0.2
0.4
E
/V vs Ag/AgCl (KCl-sat.)
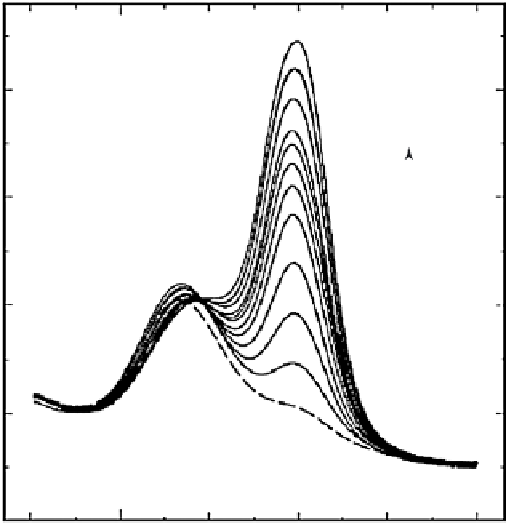
FIGURE 15.14
Osteryoung square wave voltammograms at the (PDDA/MWNT)5/GC electrode for
ascorbic acid (0.10 mM) in the presence of different concentrations of dopamine: (a) 0, (b) 2, (c) 4, (d) 6, (e)
8, (f) 10, (g) 12, (h) 17, (i) 20, (j) 24, (k) 28, and (l) 32 µM. OSWV conditions were 4 mV step height, 20 mV
pulse height, 2 Hz frequency, and 2 s quite time. (Reprinted with permission from [79]. Copyright (2005)
Elsevier.)
Figure 15.14 illustrates a typical voltammetric result for the determination of dopamine
in the presence of ascorbic acid with a CNT-modifi ed electrode. The selective voltam-
metric detection of uric acid [82] or norepinephrine [83] in the presence of ascorbic acid
has been demonstrated with a
-cyclodextrin-modifi ed electrodes incorporating CNTs.
Ye
et al.
[84] have studied the electrocatalytic oxidation of uric acid and ascorbic acid
at a well-aligned CNT electrode, which can be used for the selective determination of
uric acid in the presence of ascorbic acid. The simultaneous determination of dopamine
and serotonin on a CNT-modifi ed GC electrode has also been described [85].
It has been demonstrated that the presence of CNTs greatly increases the oxidation
peak current of 6-benzylaminopurine. The CNT-modifi ed electrode is suitable for the
determination of trace amounts of benzylaminopurine and has the advantages of high
sensitivity, quick response, and good stability [86]. Wang
et al.
have studied the electro-
catalytic oxidation of thymine at a
β
-cyclodextrin incorporated CNT coated electrode
in an alkaline media. A sensitive detection scheme for thymine has been further devel-
oped by using differential pulse voltammetry [87]. The electrochemical determination
α





Search WWH ::

Custom Search