Biomedical Engineering Reference
In-Depth Information
therefore, be interesting to focus on enzymes which oxidize fuels yet don't produce
hydrogen peroxide, such as dehydrogenases.
12.5.3 Dehydrogenases
Dehydrogenases, which represent a majority of redox enzymes, are mostly NAD
(Nicotinamide Adenine Dinucleotide, see Fig. 12.9) dependent. This cofactor is not
directly bound to the enzyme but its presence in the medium is necessary because it
acts as a carrier of two electrons and one proton, and it activates the biocatalytic func-
tion of the enzyme.
The thermodynamic redox potential of NAD
/NADH is
0.56 V vs SCE at neutral
pH. The NADH cofactor itself is not a useful redox mediator because of the high over-
potential and lack of electrochemical reversibility for the NADH/NAD
redox process,
and the interfering adsorption of the cofactor at electrode surfaces.
As dehydrogenases (DH) are widely distributed enzymes, a number of studies have
been carried out with these biocatalysts. For example, Willner
et al.
[20] have used a
PQQ-monolayer functionalized gold electrode for the catalytic oxidation of NADH in
the presence of Ca
2
. In this scheme, the pyrrolo-quinoline quinine co-factor, PQQ,
was covalently linked, as before for the GOx system [15, 20, 21], to the Au electrode,
and was capable of oxidizing NADH at
0.15 V vs SCE at pH 8.0. A theoretical
A/cm
2
is estimated for this monolayer-modifi ed system. This
method is attractive as it may be applied to oxidative reactions of the dehydrogenases
for which NAD is the cofactor.
current density of 185
µ
O
O
H
HH
C
C
NH
2
C
C
NH
2
H
C
C
C
C
C
C
C
H
C
H
N
N
H
H
CH
CH
O
O
O
O
O
O
PO
PO
NH
2
NH
2
OH
OH
OH
OH
C
C
O
O
N
N
N
N
C
C
H
C
H
C
O
O
PO
PO
C
C
C
H
C
H
H
H
N
N
N
N
O
CH
O
CH
Adenine
Adenine
O
O
Ribose
Ribose
OH
OH
OH
OH
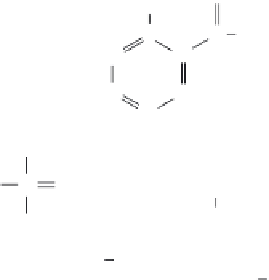
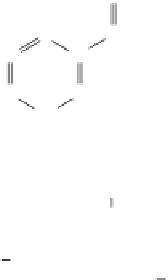
NAD
NADH
FIGURE 12.9
Structure of the NAD
/NADH cofactor.




























Search WWH ::

Custom Search