Biomedical Engineering Reference
In-Depth Information
5Mercapto 2 nitrobenzoic acid (yellow)
5,5dithio2nitrobenzoic acid (Chromogen)
AChE
Acetylcholine H
2
O
Thiocholine Acetic acid
Oxidation
2 Thiocholine
2H
2e
Dithio
bis
choline
FIGURE 2.2
Enzymatic formation of thiocholine and detection principles for pesticide.
AChE
Acetylcholine
H
2
O
Choline Acetic acid
COx
Betaine
H
2
O
2
Choline O
2
H
2
O
FIGURE 2.3
Bienzymatic reaction for pesticide detection.
the detection of sulfometuron methyl (herbicide) [19, 46]. Immobilized ALS is more
stable than the native enzyme, but the inhibition potency is higher for the free enzyme.
Polyphenol oxidase (tyrosinase) can also be irreversibly inhibited by atrazin, diazinon,
dichlorvos, etc. Besombes
et al.
[47] used an electropolymerized pyrrole/tyrosinase
coating for the detection of carbamate pesticides and reported a detection limit of 2
M
for chloroisopropylphenyl carbamate. In another study a tyrosinase electrode was con-
structed by direct adsorption of the enzyme on the surface of a graphite disc electrode.
Three carbamate pesticides, ziram, diram, and zinc diethyldithiocarbamate, inhibited
the tyrosinase in a medium of reversed micella and the detection limits of this ampero-
metric biosensor were 0.074, 1.3 and 1.7
µ
M, respectively [22]. To improve the stabil-
ity and sensitivity of a single enzyme-based biosensor the modifi ed enzymes have been
employed instead of the wild type. A genetically modifi ed
Drosophila melanogaster
AChE (DmAChE) allows its oriented immobilization on electrodes [7].
Another stable and versatile method for pesticide detection is the use of the second
enzyme, such as AChE and choline oxidase (COx). Multienzyme-based biosensors use
cholinesterase in conjunction with choline oxidase to measure hydrogen peroxide pro-
duction or oxygen consumption. COx oxidizes choline to betaine and hydrogen per-
oxide (Fig. 2.3). These methods couple an enzyme that is inhibited by the pesticide in
conjunction with another enzyme, which uses the product of the fi rst enzymatic reac-
tion as the substrate. Another bienzymatic amperometric biosensor has been reported
for the detection of dithiocarbamate fungicides [48, 49]. An aldehyde dehydrogenase
and diaphorase were coimmobilized in a PVA-SbQ layer and mounted on a screen-
printed electrode (SPE) with Pt sputtered carbon paste. The detection limits of bienzy-
matic amperometric biosensor for nabam [48] and zineb [49] were 8 and 9 ng mL
1
,
respectively. Organophosphorus and carbamate pesticides can reversibly inhibit the
catalytic activity of acid phosphatase (AP); hence AP can be reused with normal wash-
ing with buffer. But amperometric detection of AP inhibition requires a bienzymatic
system (Fig. 2.4). Mazzei
et al.
[20] immobilized AP and GOD on a separate dialytic
membrane. The bienzyme modifi ed membrane was placed on Pt electrode using an
µ


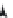










Search WWH ::

Custom Search