Biomedical Engineering Reference
In-Depth Information
1
9
N
N
N
N
Cl
N
Os
Cl
N
N
N
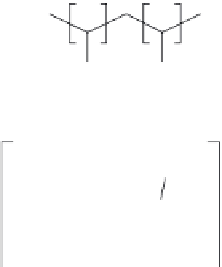
FIGURE 12.5
Structure of the osmium redox polymer, OsPVI, formed by coordination of an [Os(2,2
-
bipyridine)
2
Cl]
complex to polyvinylimidazole in a usually 1:9 ratio.
pioneered by the Heller group [54]. Although the laccase biocatalytic electrode was
developed for the reagentless detection of modulators of enzyme activity, steady-state
current densities of greater the 125
Acm
2
were achieved at potentials of
0.15 V
vs Ag/AgCl in oxygen sparged acetate buffered solutions, pH 4.5, as shown in the
cyclic voltammograms in Fig. 12.6 [55]. Subsequent to this report, this group and oth-
ers have investigated mediated laccase catalyzed reduction of oxygen in fi lms of redox
polymers on electrode surfaces for application as biocatalytic cathodes. For example,
substitution of the chloride ligand of a [Os(4,4
µ
-
terpyridine)Cl]
complex with imidazole units of PVI yields a redox polymer that
may be co-immobilized with laccase from
Coriolus hirsutus
on carbon cloth fi ber. The
resulting biocatalytic oxygen cathodes operate at mAcm
2
current densities at a poten-
tial of
-dimethyl, 2,2
-bipyridine)(2,2
:6
,2
0.7 V vs NHE in pH 5 buffer, 37ºC, when rotated at 4000 rpm [37].
Further refi nement of this cathode is also possible, by judicious choice of biocata-
lyst. The high potential fungal laccases are reportedly sensitive to chloride inhibition
and have optimal acidic pH maxima, for example, seemingly precluding their use in
physiological solutions. Co-immobilization of the redox polymer described above with
a laccase from
Pleurotus ostreatus
, which has been reported to retain a high level of
substrate oxidation activity at pH 7, yielded biocatalytic oxygen cathodes capable of
operating at
0.62 V vs NHE in pH 7, 0.1 M NaCl solution at 37ºC, with 6% of their
pH 5, chloride-free, current density [40].
The chloride and pH sensitivity of the laccase catalyzed oxygen reduction has led
some groups to focus on another class of “blue” copper enzymes, that are active under
physiological conditions of pH and NaCl, the bilirubin oxidases. Bilirubin oxidase cat-
alyzes the oxidation of bilirubin to biliverdin coupled to the four-electron reduction
of oxygen to water. The catalytic site of BOD, like laccase, consists of a tri-nuclear
T2/T3 oxygen-reducing copper site and a T1 substrate oxidizing copper site [44, 45].
Unfortunately, the reduction potential of the T1 site of the bilirubin oxidases is of the
medium potential classifi cation of
0.3 V vs Ag/AgCl [9, 10, 45]. The fi rst report
on a BOD-based biocatalytic oxygen cathode focused on homogeneous ABTS medi-
ated reduction of oxygen at carbon felt electrodes using a BOD from
Myrothecium









Search WWH ::

Custom Search