Biomedical Engineering Reference
In-Depth Information
Load
e
e
Reduced
oxidant
Cat
ox
Fuel
ox
Cat
′
Cat
red
red
Oxidized
fuel
Oxidant
Cat
′
anode
cathode
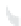
FIGURE 12.1
Schematic depiction of a biocatalytic fuel cell, with fuel oxidation by a biocatalyst (Cat)
at the anode and oxidant reduction by a biocatalyst (Cat
) at the cathode, in a membraneless assembly, pro-
viding power to the load.
consists of the modifi ed cathode and anode separated by an electrolyte containing both
fuel and oxidant, connected to a load, as depicted in the schematic in Fig. 12.1. The
design simplicity allows miniaturization of the cell. Realistic reachable power outputs,
however, should restrict future devices to powering low energy demanding systems.
12.3 ELECTRON TRANSFER REACTIONS
Electronic communication between electrode surfaces and biocatalysts can be achieved
by direct electron transfer if the active site of the biocatalyst is not located too remote
from the protein surface, as discussed elsewhere in this topic (Chapter 17). Direct elec-
tron transfer is an attractive process for fuel cells as no other molecules except the sub-
strate and the enzyme are involved in the electrocatalytic reaction, as depicted in the
schematic in Fig. 12.2. The enzyme is the relay for the electron transfer between the
substrate and the electrode surface. Recent advances in tailoring surface nanostructural
features to match the size of co-substrate channels in biocatalysts, and in reconstituting
active prosthetic groups tethered to, and communicating electronically with, surfaces,
with apo-enzymes, are elegant demonstrations of direct electron transfer to biocatalyst
active sites that were previously considered inaccessible to electrode surfaces [8-17].
Current and power densities achieved with electrodes using the direct electron trans-
fer approach will be limited, however, because of the need to have intimate contact
between the two-dimensional electrode surface and a coating monolayer of correctly
oriented biocatalyst. The use of small redox molecules that can mediate electron trans-
fer between the biocatalyst and the electrode surface offers an opportunity to improve
output from biocatalytic electrodes, as three-dimensional fi lms of biocatalysts may
now be used. In addition the distance between the active site of the enzyme and the
electrode surface is often too great to allow effi cient direct electron transfer. In these
cases the electron transfer rate is not effective because of the insulation of the redox
active site by the surrounding protein. A redox mediator can shuttle electrons between
the enzyme and the surface. In the example of redox mediated biocatalytic oxidation
of a fuel, depicted in Fig. 12.3, the enzyme catalyzes the oxidation of the mediator



















Search WWH ::

Custom Search