Biomedical Engineering Reference
In-Depth Information
X
X
OPH
(a)
R
P
O
NO
2
H
2
O
HO
NO
2
R
P
OH
R
R
OH
H
e
(b)
O
2
N
O
NO
2
HO
OH
FIGURE 2.1
Reaction scheme for OPH hydrolysis of methyl parathion and paraoxon (a) followed by the
electrochemical oxidation of p-phenol (b). R and R
are ethoxy and methoxy and X is O and S in paraoxon
and methyl parathion, respectively.
is indirectly proportional to the amount of inhibitor or pesticide in the sample. Mainly
AChE-based biosensors are superior, due to the strong enzyme inhibition by orga-
nophosphorus pesticides, enabling a higher sensitivity. The other often-employed
enzymes in pesticide biosensors are acetolactate synthase [19], acid phosphatase [20],
alkaline phosphatase [21], tyrosinase [22, 23], ascorbate oxidase [14], etc. Pesticides
can also inhibit the activity of luciferase, which is a major enzyme in bioluminescence
reactions. By employing fi refl y, luciferase pesticide concentrations have been deter-
mined, where the pesticide concentration is indirectly proportional to the biolumi-
nescence [24]. The enzyme organophosphorus hydrolase (OPH) has been used as an
alternative recognition component. OPH can hydrolyze an organophosphate molecule,
the resulting products can be monitored either spectrophotometrically or electrochemi-
cally (Fig. 2.1). Because organophosphate is the substrate for OPH, this scheme leads
to a direct determination of analyte, as the rate of signal generation is directly propor-
tional to the concentration of organophosphate [10, 25-28]. The above enzymes are
used singly; sometimes bienzymes such as AChE and choline oxidase are used [29,
30]. In the case of bienzymatic systems a number of parameters need to be optimized
and they are more complex than single enzyme systems [8].
2.2.2 Immobilization methods used in pesticide biosensors design
All the enzymes are highly selective to the substrates and sensitive to the environmen-
tal factors such as pH, temperature and inhibitors, denaturing and chelating agents.
Enzyme activity and stability on the transducer surface is governed by the procedure
followed for the immobilization and chemical nature of used matrices. The adopted
immobilization method should be suffi ciently strong to provide good mechanical sta-
bility of biosensor, and suffi ciently soft to provide optimal conformation and freedom
of the enzymes, which is crucial for reaching suffi cient enzymatic activity [31]. Several
approaches have been followed for immobilization of enzyme on the transducer/elec-
trode surface, such as adsorption [32], cross-linking with bifunctional chemical rea-
gents [33], binding with dendrimer layers [34], entrapment in different matrices [35],
including layer of cross-linked bovine serum albumin [36, 37] and electropolymeri-
zation [38], and more recently bioaffi nity attachment using concanavalin A [39], etc.













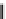







Search WWH ::

Custom Search