Biomedical Engineering Reference
In-Depth Information
0.70
7
6
5
0.65
4
3
2
1
13
µ
m
0.60
0
0.0
0.2
0.4
0.6
0.8
(Glu)/mM
1
µ
M
0.55
9
µ
M
0.50
0.4
900
1000
1100
1200
1300
Time/sec
100 nm
(a)
(b)
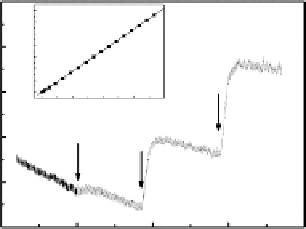
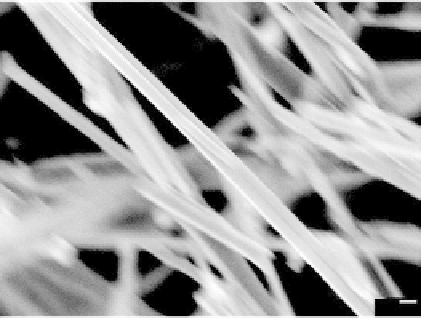
FIGURE 11.30
Zn Nanowire glucose sensor: (a) glucose oxidase impregnated Zn nanowire electrode;
(b) amperimetric response of different glucose concentration.
The most widely used examples are that of detection of glucose, based on glucose
oxidase, which generates hydrogen peroxide and gluconic acid in the presence of oxy-
gen, glucose, and water [119]. These devices are designed either for monitoring for-
mation of hydrogen peroxide or for consumption of oxygen for indirect determination
of glucose concentration. Later advances of the sensors could detect the electron trans-
fer of the glucose enzymatic oxidation by using an electron mediator [101]. Recently,
Zang and Li
et al.
reported a glucose sensor based on detection of direct electron trans-
fer by using nanostructructured zinc nanowires, which was impregnated by glucose
oxidase as shown in Fig. 11.30. The detection of direct electron transfer for the enzy-
matic oxidation can signifi cantly improve the sensing sensitivity. At the microscale,
these sensors require the formation of the working and reference electrodes on a chip,
and an enzymatic layer on the working electrode, as demonstrated for the detection of
glucose, lactose, and urea [120]. More recently, hydrogels and conducting electroac-
tive polymers have been integrated to develop electroactive hydrogels that physically
entrap enzymes within their matrices for biosensor construction and chemically stimu-
lated controlled release. Using these materials, the fabrication of glucose, cholesterol,
and galactose amperometric biosensors has been demonstrated on a chip [121].
Another representative example is electrochemical immunoassays. Electrochemical
immunoassays were introduced in the late 1970s and the methodology and applications
matured rapidly. Important advantages of such microfabricated systems as compared
with conventional systems include the ability to reproducibly handle very small amounts
of samples, improved facilities for separations of analytes, minimized dilution of the
products to be detected, and improved redox cycling effi ciencies [122]. Wang and his
coworkers [123] described microchip-based amperometric non-competitive and com-
petitive immunoassays for detection down to pg ml
1
levels of mouse IgG and
g ml
1
µ
levels of 3,3
,5-triiodo-L-thyronine, respectively, using ferrocene redox tracers (antigen
or antibodies) and capillary electrophoresis with amperometric detection. In another





Search WWH ::

Custom Search