Biomedical Engineering Reference
In-Depth Information
m/z
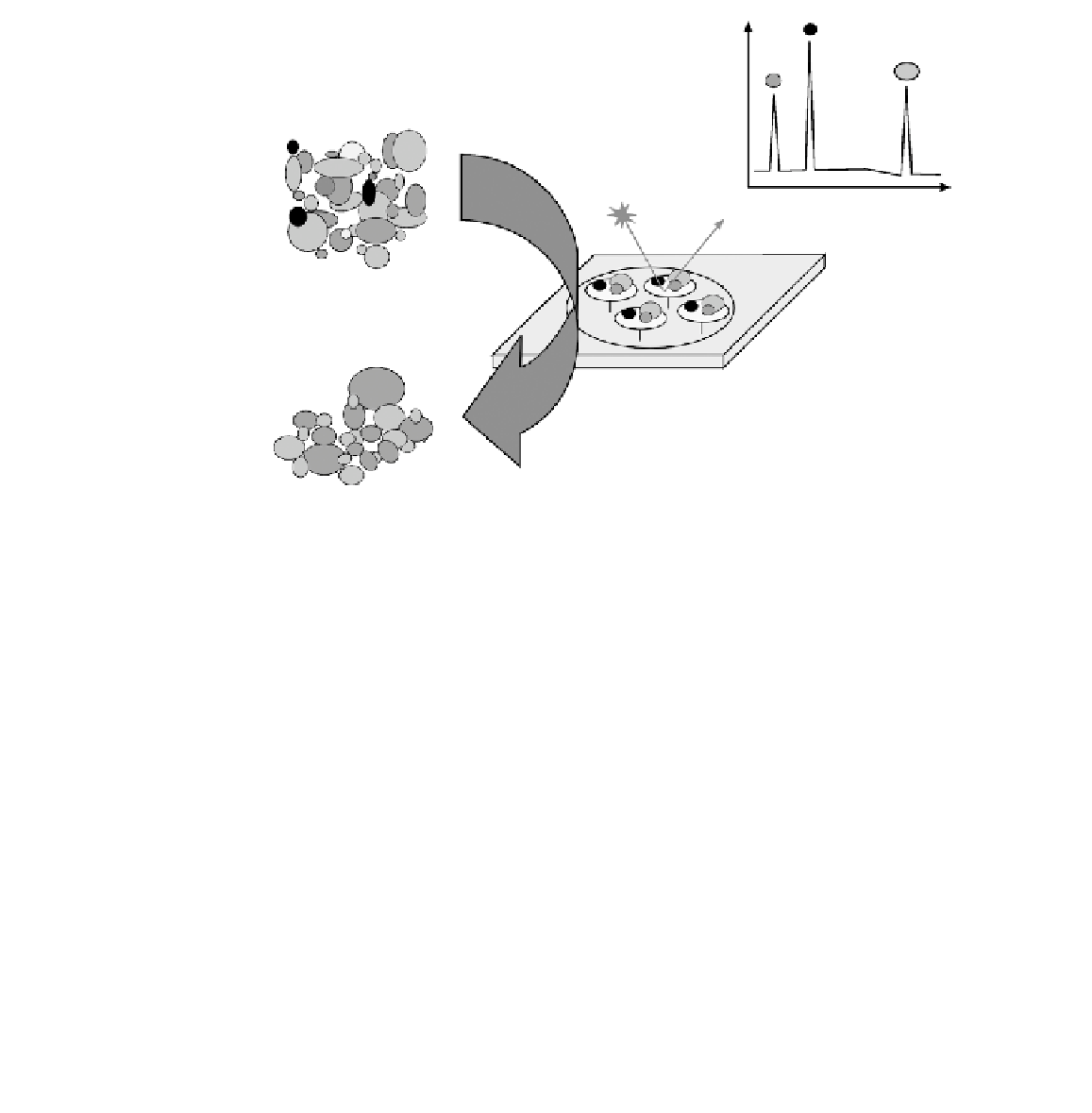
FIGURE 11.26
Schematic illustration of SELDI.
retain all relevant low affi nity interactants (to prevent false negatives) while eliminating
non-specifi c binding (to prevent false positives) is critical when working with poten-
tially hundreds of proteins simultaneously. Moreover, although macroarrays (96-well)
with polystyrene as solid phase and multi-channel spectrophotometers are commercially
available for ELISA, when it comes to microarray, the situation is more diffi cult. Efforts
should be focused on how to avoid cross-interference at different test spots caused by
diffusion of the indicator.
Undoubtedly, ELISA is a powerful tool to detect known proteins or enzyme activity.
However, this approach will never suffi ce as a method of discovering new interactants.
Protein biochips which integrate a broad range of surface affi nities with direct detec-
tion methodologies will ultimately prevail as true discovery tools. Currently SELDI
(surface enhanced laser desorption ionization)-based biochips (Fig. 11.26) are the only
commercial products that accommodate this strategy. SELDI incorporate a solid-phase
extraction adsorbent on an electrically conductive support that can be interrogated
directly by time-of-fl ight mass spectrometry (TOF-MS). The extraction adsorbents are
functionalized with a number of different chemistries to yield complementary selection
characteristics across a wide physicochemical range. Biochips can be provided as pre-
activated surfaces for the covalent binding of biomolecules, or chromatographic sur-
faces for the selective capture of proteins via charge, hydrophobicity or metal-chelate
interactions. No matter what kind of surface is utilized, the methodology for capturing
specifi c classes of proteins from a crude biological sample is similar. After incubation,
unbound or weakly bound proteins can be washed away whereas the whole variety




Search WWH ::

Custom Search