Biomedical Engineering Reference
In-Depth Information
pH
Reference
Temperature
Inner
solution
Ag/AgCl
electrode
Outer
solution
Liquid
junction
Thermocouple
pH sensitive
glass membrane
FIGURE 10.2
A schematic diagram of a combination glass pH electrode. A thin glass bulb with an inner
Ag/AgCl electrode responds to pH changes in the test solution. A second Ag/AgCl in an outer jacket with a
liquid junction serves the reference electrode for potentiometric measurement. An attached temperature probe
is used to compensate for temperature effects.
When the glass membrane is immersed in an aqueous solution, a thin hydrated layer
(
m) is formed on the glass-solution interface, depending on the compo-
sition of the glass and soak environments [56]. Monovalent ions such as Na
from the
glass exchange with the H
ions of the solution in a diffusion process. At equilibrium,
there is a build-up of charge, therefore a potential is established across the membrane
that is proportional to the hydrogen ion concentration in the test solution. According
to the Nernst equation as described in Eqs (3) and (4), the glass electrode potential is
related to the pH as follows:
10 nm to 10
µ
E
k
59 (mV/pH) pH
(6)
where
k
is a constant and the slope 59 (mV/pH) is calculated at 25ºC.
Generally accepted theories about the origin of the pH response of glasses are based
on the assumption that hydrogen ions do not cross the glass membrane. Studies reported
by Abe and Maeda [57] suggest that some mobile hydrogen ions exist in glass and are
responsible for pH response. The diffusion coeffi cient of hydrogen ions in pH sensitive
glass membranes is estimated based on a stimulated model and experimental data to be
1
10
8
cm
2
/s [58]. This value might be overestimated as it is three orders of magni-
tude higher than that of non-plasticized membranes (10
11
to 10
12
cm
2
/s) but in the
same order of magnitude as the pH ion carrier in ionophore doped plasticized polymer
membranes [59].
The pH sensing glass consists of mainly SiO
2
with some non-silicon components.
The most widely known example of pH sensitive glass is Corning 015, which contains

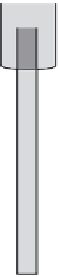


















Search WWH ::

Custom Search