Biomedical Engineering Reference
In-Depth Information
10
0
10
6
8
10
pH
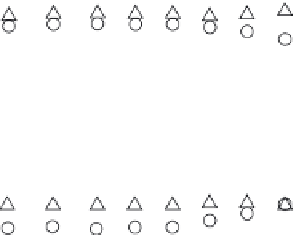
FIGURE 6.12
Plots of amperometric responses of Cu, Zn-SOD/MPA-modifi ed ( ), Mn-SOD/MPA-
modifi ed ( ), and Fe-SOD/MPA-modifi ed ( ) Au electrodes toward 13 nM min
1
O
2
•
in 25 mM phosphate
buffer at various pH values from pH 5.8 to 9.5. The solution was stirred with a magnetic stirrer at 200 rpm.
(Reprinted from [138], with permission from the American Chemical Society.)
UA, AA, and DOPAC, with the concentrations approximating their extracellular
fl uid levels were investigated at
300 and
100 mV at the SOD-based biosensors;
at
300 mV, the interferences from AA and UA were considerable, for instance 15%
and 23% current responses were obtained for 500
M AA relative to 13 nM O
2
•
with
Fe-SOD/MPA-modifi ed and Mn-SOD/MPA-modifi ed Au electrodes, respectively. In
addition, 10% current response was obtained for 50
µ
M UA relative to 13 nM O
2
•
at both electrodes. Fortunately, such interferences were well suppressed when the
electrodes were polarized at
µ
100 mV. Besides, the interferences of H
2
O
2
,
5-HIAA, HVA, and DOPAC were negligible at both
300 and
100 mV at both
electrodes [138].
On the other hand,
in-vivo
formation of physiologically inappropriate levels of free
radicals occurs in response to low blood fl ow, low oxygen levels, and low pH [154,
155]. The probable interference from pH and O
2
was consequently investigated over
the biologically relevant range. Figure 6.12 shows the steady-state amperometric
responses for O
2
•
at the SOD-based biosensors at various pH values. It should be
noted here that the rate of O
2
•
generation in the xanthine-XOD system depends on
solution pH because of the pH dependence of the enzymatic activity of xanthine oxi-
dase. Therefore, the rate of O
2
•
generation under various pH values was determined
by recording the reduction of ferricytochrome
c
spectrophotometrically and using the
extinction coeffi cient (21.1 mM
1
cm
1
) of ferrocytochrome
c
at 550 nm to guarantee













Search WWH ::

Custom Search