Biomedical Engineering Reference
In-Depth Information
Cu, Zn-SOD
1.25 nA
300 mV
SOD (M
2/3
)
O
2
10 s
e
(a)
SOD (M
/2
)
O
2
Xanthine
100 mV
(b)
SOD (M
2/3
)
H
2
O
2
e
SOD (M
/2
)
O
2
Catalase
Cu, Zn-SOD
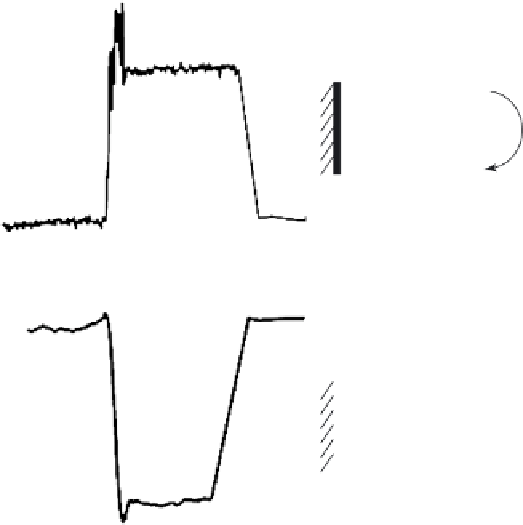
FIGURE 6.11
Typical current-time responses of Fe-SOD/MPA-modifi ed Au electrode toward O
2
•
in
25 mM phosphate buffer (O
2
-saturated, pH 7.5) containing 0.002 unit of XOD upon the addition of 50 nM
xanthine at
300 (a) and
100 mV (b). The arrows represent the addition of 10
µ
M of Cu, Zn-SOD (a)
and 580 units of catalase and 10
M of Cu, Zn-SOD to the solution (b). The solution was stirred with a
magnetic stirrer at 200 rpm. Inset: mechanism for the amperometric response of SODs/MPA-modifi ed Au
electrodes to O
2
•
based on enzymatic catalytic oxidation (a) and reduction (b) of O
2
•
(M: metal ions of
SODs). (Reprinted from [138], with permission from the American Chemical Society.)
µ
of the SODs for the dismutation of O
2
•
; namely, these SODs catalyze both the reduction
of O
2
•
to H
2
O
2
and the oxidation to O
2
via a redox cycle of active metals as shown in
Scheme 3, and on the direct electron transfer of the SODs realized at the MPA-modifi ed
Au electrode as described in the above sections. These demonstrations reveal that, simi-
lar to the bifunctional catalytic activity observed for Cu, Zn-SOD, the Fe-SOD and
Mn-SOD also possess the bifunctional electro-catalytic activity toward O
2
•
.
Figure 6.11, with the Fe-SOD/MPA-modifi ed Au electrode as an example, displays
a typical amperometric response of the electrode toward O
2
•
. A large anodic current
was recorded at the Fe-SOD/MPA-modifi ed Au electrode at
300 mV when xanthine
(50 nM) was introduced into the phosphate buffer solution to generate O
2
•
(a), while
relative small responses were obtained at the bare Au electrode and the MPA-modifi ed
Au electrode for the same concentration of O
2
•
(not shown), indicating the enzymatic
amplifi cation nature of O
2
•
oxidation at the Fe-SOD/MPA-modifi ed Au electrode. The
assignment of the observed large anodic current to the oxidation of O
2
•
, rather than




































Search WWH ::

Custom Search