Biomedical Engineering Reference
In-Depth Information
300
c
200
b
100
a
0
6
8
10
pH
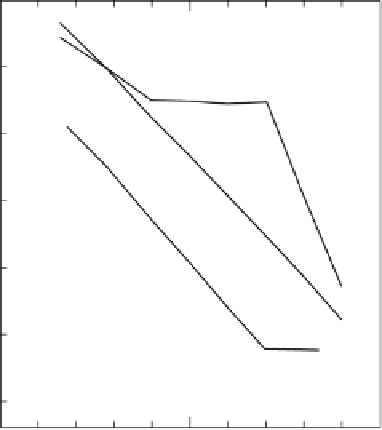
FIGURE 6.6
Plots of the formal potentials of (a) Fe-SOD, (b) Cu, Zn-SOD, and (c) Mn-SOD at the
MPA-modifi ed Au electrode vs solution pH. (Reprinted from [138], with permission from the American
Chemical Society.)
“MPA-bridged SOD-electrode complex” could be formed via a variety of interactions
between MPA and the SODs, such as electrostatic, hydrophobic, and/or hydrogen
bonding interactions, which is believed to be responsible for the observed direct elec-
tron transfer properties of the SODs. Besides, such interactions substantially enable the
SODs to be stably confi ned at the MPA-modifi ed Au electrode, which can be further
evident from the re-observation of the redox responses of SODs in a pure electrolyte
solution containing no SOD with the MPA-modifi ed electrode previously used in SOD
solutions.
The formal potentials (
E
0
) of the three kinds of SODs were found to be depend-
ent on solution pH as displayed in Fig. 6.6. As shown, the formal potential of bovine
erythrocyte Cu, Zn-SOD decreases linearly with increasing solution pH with a slope
of ca.
60 mV/pH from pH 5.8 to pH 9.5 (curve b), indicating one proton and one
electron are included in the electrode reaction of Cu, Zn-SOD, which is similar to pre-
viously proposed enzymatic catalytic mechanistic scheme of the Cu, Zn-SOD [139-
144]. In contrast, the pH dependency of Fe-SOD from
E. coli
was complicated (curve
a); the formal potential changes linearly with solution pH in a range from pH 5.8 to
8.5 with a slope of ca.
8.5.
Previous studies have observed that the Fe (III) form of the protein ionizes with an
apparent p
K
a
of 9.0
60 mV/pH, and becomes pH-independent at above pH
0.3 and such ionization effect has been interpreted in terms of
hydrolysis of a bound water molecule with p
K
a
of ca. 8.5 [145]. The
E
0
-pH profi le of






























Search WWH ::

Custom Search