Biomedical Engineering Reference
In-Depth Information
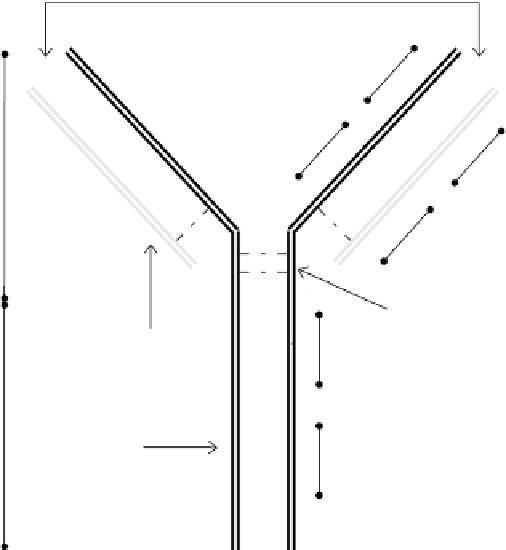
divided into constant (C) and variable (V) domains based on their amino acid sequence
variability. The light chains have a single variable domain (V
L
) and a single constant
domain (C
L
). In comparison, a heavy chain consists of a single variable domain (V
H
)
and three constant domains (C
H
1, C
H
2, C
H
3). In general, the antibody molecule may
be divided into two main fragments, the non-antigen binding fragment (denoted as Fc)
and the antigen-binding fragment (F(ab
)
2
), as indicated in Fig. 5.1.
The variable domains in both chain types are the most important regions with regard
to the antibody-antigen binding interaction. The specifi city of an antibody towards
the binding site (or epitope) of its antigen is a function of its amino acid sequence.
Within the V
L
and V
H
domains, there are three distinct subregions of high sequence
variability, known as hypervariable regions. There are three on each light chain and
three on each heavy chain, forming six hypervariable loops known as complementa-
rity determining regions, which constitute the antigen binding site. It is the diversity
in this region that allows antibodies of high affi nity to be produced against almost any
antigen. It is estimated that 10
8
antibody specifi cities can be produced from this one
basic molecular structure, and an individual antibody will usually recognize only one
antigen, although there are possible cross-reactivities [8].
antigen binding sites
V
H
C
H
1
F(ab
)
2
′
V
L
C
L
ss
s
s
hinge region
(disulfide linkages)
C
H
2
light chain
Fc
heavy chain
C
H
3
FIGURE 5.1
A schematic illustrating the “Y”-shaped structure of an antibody. The region between the
heavy chain and the light chain is where antigen binding occurs. This open arm portion of the “Y” shape is
generally denoted as F(ab
)
2
, while the non-antigenic binding site in the base portion is referred to as Fc.





Search WWH ::

Custom Search