Biomedical Engineering Reference
In-Depth Information
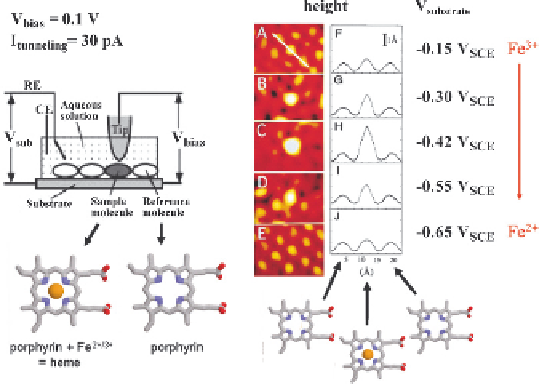
the Fe-containing molecules when the Fermi levels of the source
and drain electrodes (the substrate and tip, held at constant bias of
0.1 V) aligned with the lowest unoccupied molecular orbital in
Fe(III). This resonant effect disappeared again when the potentials
of the electrodes were set more negative than the redox potential of
the Fe-containing molecules (
Fig. 7
).
In the years to follow, several groups have similarly applied
electrochemical STM, observing comparable resonant effects with
other types of redox molecules and, since the earliest days of the
field, with redox-active protein molecules. The examples are too
numerous to be summarized here and have already been extensive-
ly reviewed elsewhere.
153,156
Along with experimental work, the
theoretical underpinning was developed (summarized in the re-
views cited in the introduction of junction devices), e.g., models
Figure 7. The first experimental demonstration of electrochemical resonant tunnel-
ing enhancement. Left: Schematic
in-situ
STM cell design (RE = Reference Elec-
trode, CE = Counter Electrode), with both FE(III)-protoporphyrin IX and metal-
free protoporphyrin IX on the substrate electrode. Right: Resonant tunneling due to
the redox-active Fe(III) (reduction potential -0.48 V vs. SCE. Adapted with per-
mission from Ref. 161, Copyright (1996) American Physical Society.
Search WWH ::

Custom Search