Biomedical Engineering Reference
In-Depth Information
8
1
BFg
0
-1
4
-2
Control
10 min
60 min
-3
0
-4
-4
Control
10 min
60 min
1 µm
200 nm
-8
1
4
γ-Ig
0
-1
2
-2
-3
0
-4
-2
-4
1 µm
200 nm
-6
0 75 150 nm
0 25 50 nm
12
1
0
Tf
-1
6
-2
-3
0
-4
-6
-12
200 nm
200 nm
45
0
30
BSA
-1
-2
15
-3
-4
260
280 300
nm
320 340 360
0
-15
200 nm
200 nm
-30
190
200
210
220
230
240
(a)
(b)
(c)
(d)
(e)
(f)
(g)
0
10 20 nm
0 10 20 nm
nm
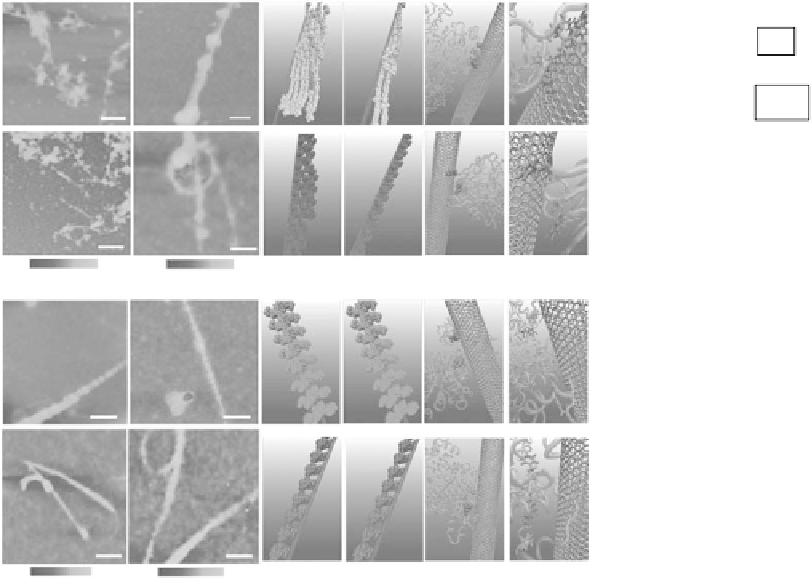
Figure 27.5
(See companion CD for color igure.)
. Interactions. between. human. blood. proteins. (BFg,. γ-Ig,. Tf,. BSA). and.
SWCNTs..AFM.images.of.proteins.after.incubation.with.SWCNTs.for.10.min.(a).and.5.h.(b)..Molecular.modeling.
illustrations. for. proteins. (in. beads. representation). binding. to. SWCNTs. after. incubation. for. 10.min. (c). and. 5.h.
(d)..(e).Locations.of.the.most.preferred.binding.sites.on.proteins.for.SWCNTs..Residues.highlighted.in.Van.der.
Waals.representation.corresponding.to.tyrosine.colored.in.red.and.phenylalanine.colored.in.green..Other.parts.
of.protein.are.represented.in.transparent.pink.with.the.new.cartoon.drawing.method..(f).The.detailed.orienta-
tions.of.aromatic.rings.of.tyrosine.and.phenylalanine.residues.interacted.with.six-member.rings.of.SWCNTs,.
colored.in.silver..The.tyrosine.residues.are.rendered.as.a.Licorice.representation.and.colored.in.red,.with.phenyl-
alanine.residues.in.green..(g).The.far-UV.CD.spectra.of.proteins.after.incubation.with.SWCNTs.and.the.insets.
are.near-UV.CD.spectra.of.proteins.incubated.with.SWCNTs..(Reproduced.by.permission.from.Ge,.C.,.Du,.J.,.
Zhao,.L.,.Wang,.L.,.Liu,.Y.,.Li,.D.,.Yang,.Y..et.al.,.Binding.of.blood.proteins.to.carbon.nanotubes.reduces.cyto-
toxicity..
Proc. Natl. Acad. Sci. USA
,.108,.16968-16973,.2011a..Copyright.2011.National.Academy.of.Sciences,.USA.)
surface. of. SWCNTs,. which. is. governed. by. each. protein's. unique. structure. and. amount.
of.hydrophobic.residues.(Figure.27.5)..Atomic.force.microscopy. (AFM).images. indicated.
that.the.adsorption.of.transferrin.(Tf).and.bovine.serum.albumin.(BSA).quickly.reached.
thermodynamic. equilibrium. in. only. about. 10.min,. while. ibrinogen. (BFg). and. gamma-
globulin. (γ-Ig). gradually. packed. onto. the. SWCNT. surface. over. a. much. longer. period. of.
about. 5.h.. Both. luorescence. spectroscopy. and. Sodium. dodecyl. sulfate. polyacrylamide.
gel.electrophoresis.SDS-PAGE.have.shown.surprising.competitive.adsorptions.among.all.
the.blood.proteins.examined,.with.a.competitive.order:.BFg.>.γ-Ig.>.Tf.>.BSA..The.far-UV.
CD. spectra. observation. also. shows. that. the. protein. secondary. structure. has. changed.
signiicantly. for. BFg. and. γ-Ig,. with. a. decrease. in. the. α-helical. content. and. an. increase.
in.the.β-sheet.structure.(Figure. 27.5g).. In.addition,. our. molecular. dynamics. simulations.
on.SWCNTs.binding.with.BFg,.BSA,.γ-Ig,.and.Tf.complexes.showed.that.both.the.contact.