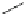Biomedical Engineering Reference
In-Depth Information
are highly valuable for providing functional amphiphilic biodegradable polyester-
based materials. In other words, PDPs have both the functionality of polypeptides
and the degradability of polyesters. These polymers contain both ester and amide
groups in the chain, so their biodegradation behavior is different to that of the
homopolymers.
Initially PDPs were synthesized by stepwise polycondensation of linear
activated depsipeptide [
93
]. In 1985, Helder, Feijen and coworkers reported the
synthesis of PDPs by ROP of a morpholine-2,5-dione derivative (cyclic dimer of
a
-hydroxy- and
a
-amino acid; cyclodepsipeptide, cDP) [
94
,
95
]. The ROP method
gives an alternative type of PDP by homopolymerization and also allows the
copolymerization with other monomers (lactones and cyclic diesters) including
LA, GA, and CL to give a wide variety of functional biodegradable materials.
The synthesis of PDPs as functional biomaterials has been recently reviewed [
17
].
Several groups have tried to polymerize 3-alkyl-substituted morpholine-
2,5-dione derivatives (3-alkyl substituted cDPs, i.e.,combinations of amino acids
with alkyl side chains with glycolic acid or lactic acid) to synthesize aliphatic
PDPs [
96
-
99
]. After these studies, the syntheses of PDPs and poly(DP-
co
-LA)
s with reactive (hydrophilic) functional groups using amino acids (Asp, Glu, Lys,
Cys, Ser) with protected reactive side-chain groups (-COOH, -NH
2
, -SH, -OH),
and subsequent deprotection, were reported by some groups including one of the
authors (Fig.
4
)[
100
-
107
]. Using these methods, aliphatic polyesters having reac-
tive side-chain groups can be produced and utilized in various applications such as
DDS and tissue engineering using the reactivity and hydrophilicity of the side-chain
groups. Langer et al. reported copolymerization of cDP containing Lys as amino
acid to give poly(DP-
co
-LA) [poly(Lys-LA)], and immobilization of RGD peptide
on the PLA-based materials for a biodegradable cell-adhesive scaffold for tissue
H
O
1. polymerization
2. deprotection
O
N
O
O
O
R'
R
O
N
H
n
polydepsipeptide
cyclodepsipeptide
H
CH
3
O
O
1. polymerization
2. deprotection
O
N
O
O
O
O
R
O
CH
3
O
n
O
O
poly(depsipeptide-
co
-lactide), P(DP-co-LA)
L-lactide
R = (CH
2
)
4
NH
2
: poly[(Glc-Lys)-
co
-LA]
CH
2
COOH: poly[(Glc-Asp)-
co
-LA]
(CH
2
)
2
COOH: poly[(Glc-Glu)-
co
-LA]
(CH
2
)SH: poly[(Glc-Cys)-
co
-LA]
CH
2
OH: poly[(Glc-Ser)-
co
-LA]
Fig. 4 Synthesis of polydepsipeptides and poly(depsipeptide
-co
-lactide)s having reactive
side-chain groups