Biomedical Engineering Reference
In-Depth Information
electrostatic (coulombic) interactions. Nanoscaled structural materials (e.g.,
nanoparticles, micelles, nanogels, and hollow nanospheres) composed of PIC
are prepared by tuning the preparation conditions, such as the charge ratio of the
anionic-to-cationic polymers, temperature, concentration, and type of polyelectrolyte
[
12
,
86
,
87
].
PIC containing
g
-PGA and chitosan (CT) as a cationic polymer has been used for
preparation of nanoparticles, hydrogels, and films for biomedical applications. Sung
et al. investigated the PIC particle formation of
g
-PGA and CT by self-assembly
in aqueous media [
88
]. Nanoparticles were obtained upon addition of a
g
-PGA
(160 kDa) aqueous solution (pH 7.4) into a low molecular weight CT (50 kDa)
aqueous solution (pH 6.0). It was found that the particle size and the zeta potential of
the prepared nanoparticles were mainly determined by the relative amount of the
local concentration of
g
-PGA in the added solution to the surrounding concentration
of CT. The size (80-400 nm) and surface charge (from
35 to +25 mV) of
g
-PGA-
CT nanoparticles could be easily controlled by changing the mixing ratio of two
polymers. Hajdu et al. also prepared
g
-PGA (1,200 kDa)-CT (320 kDa)
nanoparticles [
89
]. The size and size distribution of the nanoparticles depended
on the concentrations of
g
-PGA and CT solutions and their ratio as well as on the pH
of the mixture and the order of addition. The particle size was in the range of
20-285 nm, as measured by transmission electron microscopy (TEM), and
the average hydrodynamic diameters were between 150 and 330 nm.
The stability and characteristics of prepared PIC are influenced by various
factors involving their chemical compositions and their surrounding environment.
In particular, for PIC micelles or nanoparticles, the ionic strength and pH of the
solution is a key parameter for stability because of the shielding effect of the
ionic species on the electrostatic interactions [
90
]. Therefore, destabilization of
PIC under physiological conditions limits their applications as a drug carrier. For
the development of stable PIC nanoparticles under physiological conditions,
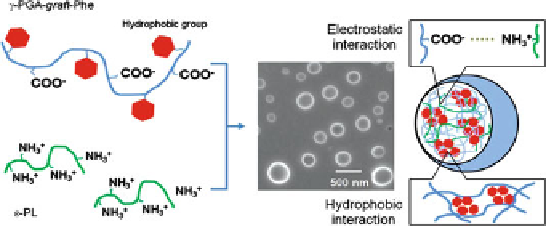
Akagi et al. focused on a novel approach for the stabilization of PIC nanoparticles
by hydrophobic interactions. Amphiphilic
g
-PGA-Phe as the biodegradable
anionic polymer, and
e
-PL as the cationic polymer were used for preparation of
PIC nanoparticles (Fig.
9
)[
91
]. The PIC nanoparticles were prepared by mixing
Fig. 9 Stabilization of polyion complex nanoparticles composed of poly(amino acid)s using
hydrophobic interactions