Biomedical Engineering Reference
In-Depth Information
2 Preparation of Biodegradable Polymeric Nanoparticles
2.1 PLGA Nanoparticles
Biodegradable polymeric nanoparticles have attracted much attention for their
potential in biomedical applications, such as drug, gene, and vaccine delivery
systems. The biodegradation rate and the release kinetics of loaded drugs can be
controlled by the composition ratio and the molecular weight of the polymer and
block/graft copolymers [
26
-
28
]. Furthermore, by modulating the polymer
characteristics, one can control the release of a therapeutic agent from the
nanoparticles to achieve a desired therapeutic level in a target tissue for the required
duration for optimal therapeutic efficacy. The commonly used biodegradable
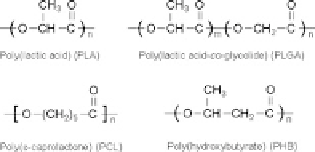
polymers are aliphatic polyesters such as poly(lactic acid) (PLA), poly(glycolic
acid) (PGA), poly(
e
-caprolactone) (PCL), poly(hydroxybutyrate) (PHB) and their
copolymers (Fig.
2
)[
29
]. In particular, poly(lactide-
co
-glycolide) (PLGA) has been
the most extensively investigated for developing nano- and microparticles
encapsulating therapeutic drugs in controlled release applications [
30
-
32
]dueto
their inherent advantages. The copolymers have the advantage of sustaining the
release of the encapsulated therapeutic agent over a period of days to several weeks.
As polyesters in nature, these polymers undergo hydrolysis upon administration
into the body, forming biologically compatible and metabolizable moieties (lactic
acid and glycolic acid) that are eventually removed from the body by the citric
acid cycle.
Several methods have been reported for the preparation of biodegradable
nanoparticles from PLGA, PLA, and PCL by dispersing preformed polymers.
Emulsion solvent evaporation techniques are frequently used to prepare nano-
and microparticles [
33
,
34
]. The polymer is dissolved in an organic solvent like
dichloromethane, chloroform, or ethyl acetate and then emulsified into an aqueous
solution to create an oil-in-water (o/w) emulsion by using a surfactant such as poly
(vinyl alcohol). After the formation of a stable emulsion, the organic solvent is
evaporated by increasing the temperature under pressure (Fig.
3
). The effect of this
process is variable, depending on the properties of the nanoparticles. Often,
surfactants are used to stabilize the nanoparticles in aqueous solution in order to
prevent the aggregation and/or precipitation of water-insoluble polymers. However,
adequate removal of the surfactant remains a problem, and surfactant molecules are
sometimes harmful in biomedical applications.
Fig. 2 Chemical structures
of biodegradable polyesters
used for preparation of
nanoparticles