Biomedical Engineering Reference
In-Depth Information
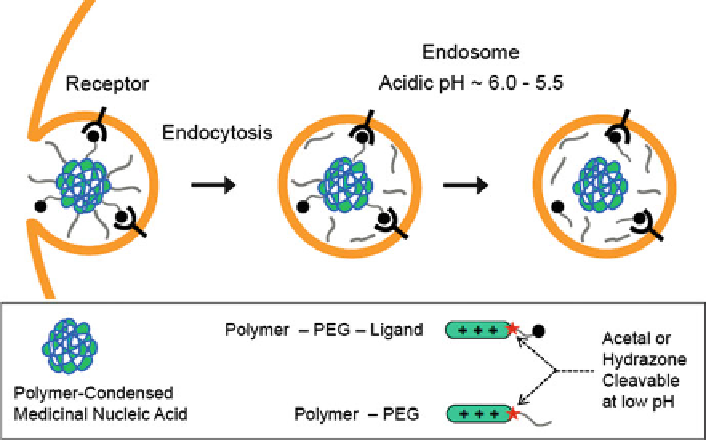
Fig. 2 Deshielding of polyplexes. After endocytosis of polyplexes into endosomes, deshielding
by cleavage of PEG hydrazone or acetal linkers
incorporated into PEG-polymer conjugates. Deshielding at endosomal pH strongly
(up to 100-fold) enhanced gene transfer of targeted PEG-PEI/pDNA polyplexes
in vitro and in vivo [
69
,
76
]. The most plausible explanation for this positive effect
is cleavage of the PEG inside the endosomes, exposing cationic PEI domains
with endosomolytic properties. In analogous fashion, DNA/PEI lipopolyplexes
containing PEG linked with the lipid layer via pyridyl hydrazone linkages were
far more effective than their pH-stable analogs [
201
]. Dynamic siRNA polycon-
jugates [
56
] contain an endosomal-sensitive dialkylmaleic acid linkage between a
cationic amphipathic (butyl-amino-modified) polyvinyl ether and PEG.
A different pH-triggered deshielding concept with hydrophilic polymers is
based on reversing noncovalent electrostatic bonds [
78
,
195
,
197
]. For example, a
pH-responsive sulfonamide/PEI system was developed for tumor-specific pDNA
delivery [
195
]. At pH 7.4, the pH-sensitive diblock copolymer, poly(methacryloyl
sulfadimethoxine) (PSD)-
block
-PEG (PSD-
b
-PEG), binds to DNA/PEI polyplexes
and shields against cell interaction. At pH 6.6 (such as in a hypoxic extracellular
tumor environment or in endosomes), PSD-
b
-PEG becomes uncharged due to
sulfonamide protonation and detaches from the nanoparticles, permitting PEI to
interact with cells. In this fashion PSD-
b
-PEG is able to discern the small difference
in pH between normal and tumor tissues.
Tumor tissues overexpress matrix metalloproteinases (MMPs). A liposomal
pDNA carrier (MEND) was developed containing PEG conjugated to lipid via a
peptide linker that is a target sequence for MMPs. In this strategy, PEG is removed
from the carrier via MMP-triggered cleavage [
198
]. Intravenous administration in