Biomedical Engineering Reference
In-Depth Information
copolymers with
a
-CDs were investigated by X-ray diffraction and solid-state
13
C CP/MAS (cross-polarization/magic angle spinning) NMR spectroscopic
methods. The results suggested a channel-type crystalline structure with CDs due
to the long chain nature of the triblock copolymers. We confirmed enantiospecific
recognition of chiral PLLA by
a
-CD as a low molecular weight chiral host on
pPRX formation [
291
]. PLLA could effectively form pPRX with
a
-CD, but PDLA
did not.
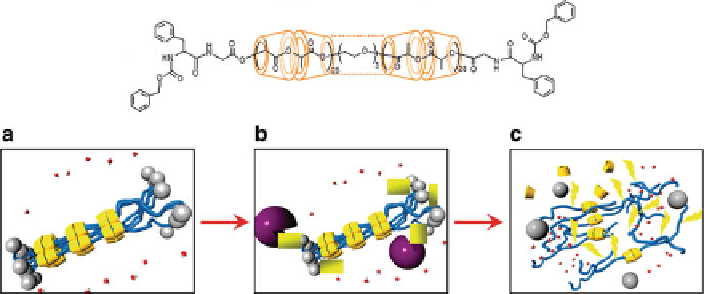
We further synthesized biodegradable PRX composed of PLLA and
a
-CD
(LA-PRX) by capping reaction of an amino group introduced at the termini of
main chain PLLA [
292
]. The end-capping groups were attached through enzymati-
cally degradable peptide linkages. Then, we investigated the enzymatic degradation
behavior of the LA-PRX synthesized by each of the two strategies (using biode-
gradable links between main chain and end-capping groups or using biodegradable
main chain). The PLLA chain in the obtained LA-PRX showed slower degradation
behavior in the absence of papain, but rapidly degraded in the presence of papain
compared with naked PLLA. These results mean that CDs can act as hydrolysis
inhibitors in PRX form. However, cleavage of end-capping groups by papain and
subsequent release of CDs led to rapid degradation of PLLA main chain (Fig.
19
).
Therefore, the LA-PRX system is expected to be applied to the development of
biodegradable medical devices exhibiting specific stimuli-responsive degradation,
drug release, or time-controlled excretion.
Zhuo and coworkers recently reported supramolecular hydrogels based on PRX
[
293
]. They encapsulated several kinds of cells in the hydrogel. The in vitro
cytotoxicity and histological studies demonstrated good biocompatibility and
suggested that the hydrogels were good candidates for injectable scaffolds for
tissue engineering and drug delivery devices.
Fig. 19 Structure of LA-PRX (
above
) and degradation of LA-PRX (
below
). (a) Threaded
a
-CDs
prevent hydrolysis of PLLA in LA-PRX. (b) LA-PRX converts into LA-pPRX by peptide linkage
cleavage at bulky end-capping groups through action of papain. (c) Ester bond hydrolysis in the
PLLA chain begins by an exposure of PLLA to water by release of
a
-CDs from LA-pPRX.
Reprinted from [
292
] with permission