Biomedical Engineering Reference
In-Depth Information
as compared to the best head of series RPR-120535. On the other
hand, when the C-18 lipid chain in RPR-120535 was replaced by
shorter chains of C-14, C-13, or C-12, a significant lose in transfection
efficiency of one, two, or three orders was observed concomitantly
[20].
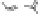
Another novelty of this approach was the introduction of a fou-
rth, new element in the lipopolyamine backbone called “side chain
entity.” This side chain could play various roles such as targeting,
labeling, or stabilizing lipid/DNA complexes. This approach was
demonstrated by the synthesis of a series of lipopolyamines bearing
a variety of side chains suitable for targeting (see Fig. 1.5) (biotinyl
[RPR-122761], arachidonyl [RPR-130605], and glycosyl [RPR-
130596]), for labelling (rhodaminyl [RPR121653]), or for physico-
chemical stabilization of complexes with DNA (guanidyl [RPR120531
and RPR121650]). Thus, it was demonstrated that the introduction
of a linker bearing a side chain entity is allowed for transfection
in
vitro
. Additionally, the introduction of a molecular probe,
such as rhodaminyl, allowed to investigate the intracellular fate of
DNA/cationic lipid complexes [27].
and
in vivo
O
H
H
O
H
H
N
N
N
N
N
N
H
N
N
N
N
2
H
H
H
N
N
O
2
H
H
O
RPR130605
O
N
H
H
N
S
RPR122761
O
H
N
N
H
O
O
O
H
H
H
H
N
N
N
N
N
N
H
N
N
N
H
N
N
N
2
H
H
2
O
H
H
O
H
O
O
H
RPR130596
O
H
O
N
RPR121653
H
N
S
H
O
H
O
C
O
O
-
+
N
N
O
H
H
O
O
H
H
N
N
N
N
N
N
H
N
N
N
H
N
N
N
2
H
H
2
O
H
H
O
H
RPR120531
H
N
RPR121650
O
N
N
H
N
O
N
H
N
H
2
O
O
Figure 1.
Introduction of side chain entities into lipopolyamines.



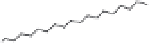
















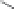












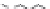




















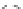








































































Search WWH ::

Custom Search