Biomedical Engineering Reference
In-Depth Information
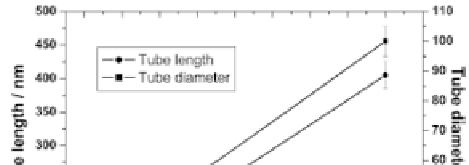
combined with tube length from 20 nm to 1 μm [4]. The diameter
and the length depend linearly on the voltage, too. So, by careful
choice of anodic oxidation parameters, it is possible to control the
growing nanotubes.
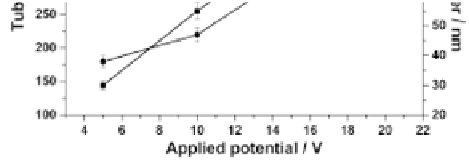
Figure 9.50
Dependence of the tube diameter and length on the applied
potentials [53].
Yang
et al
. [111] showed the formation of nanotubular Ti on
sputtered material (Fig. 9.51). They deposited titanium layers of
863 nm thickness on silicon substrates by DC magnetron sputtering.
The anodization procedure consisted etching in aqueous solution
(1M Na
2
SO
4
+ 0.2M C
6
H
8
O
7
· H
2
O with the addition of 0.4 wt%
NaF) and glycerol electrolyte (0.5 wt% NH
4
F in glycerol). The
potential was ramped by 0.1 V/s from the ocp to 5-30 V and
then kept at constant value for various times. After anodization,
the standard rinsing with deionized water and drying with a
nitrogen stream was applied. Much higher current densities are
observed in the aqueous electrolyte than in the glycerol electrolyte,
because higher diffusivity and concentration of ions in the aqueous
solution. The nanotubes are open on the top and closed at the
bottom [111]. The morphologies of nanotube anodized in glycerol
electrolyte were different from those in aqueous electrolyte
(Fig. 9.51). The outer diameter of the nanotubes increases with
the increasing anodization voltage for both types of electrolytes. The
nanotube diameter was remarkably affected by the electrolyte and
at the same applied voltage; the nanotubes with 100 nm and 40 nm
diameters were obtained in the aqueous and glycerol electrolyte,
respectively.